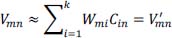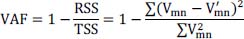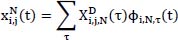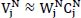Beltrame G., Scano A., Marino G., Peccati A., Molinari Tosatti L., Portinaro N. (2023) Recent developments in muscle synergy analysis in young people with neurodevelopmental diseases: A systematic review. Frontiers in Bioengineering and Biotechnology 11, 1145937. |
 Chang S., Hu C. (2025) Comparative effects of narrow vs. wide cuff blood flow restriction on muscle synergy dynamics: A time-frequency decomposition approach. Sensors (Basel) 25. |
 Chang Y.H., Roiz R.A., Auyang A.G. (2008) Intralimb compensation strategy depends on the nature of joint perturbation in human hopping. Journal of Biomechanics 41, 1832-1839. |
 Comfort, P., Jones, P.A. and McMahon, J.J. (2018) Performance
assessment in strength and conditioning. Routledge. |
 Cornejo-Daza P.J., Sánchez-Valdepeñas J., Rodiles-Guerrero L., Boullosa D., León-Prados J.A., Wernbom M., Pareja-Blanco F. (2025) Training effects of traditional versus cluster set configuration with and without blood flow restriction. Medicine & Science in Sports & Exercise 57, 668-679. |
 Del Vecchio A., Negro F., Holobar A., Casolo A., Folland J.P., Felici F., Farina D. (2019) You are as fast as your motor neurons: Speed of recruitment and maximal discharge of motor neurons determine the maximal rate of force development in humans. The Journal of Physiology 597, 2445-2456. |
 Ellefsen S., Hammarström D., Strand T.A., Zacharoff E., Whist J.E., Rauk I., Nygaard H., Vegge G., Hanestadhaugen M., Wernbom M., Cumming K.T., Rønning R., Raastad T., Rønnestad B.R. (2015) Blood flow-restricted strength training displays high functional and biological efficacy in women: A within-subject comparison with high-load strength training. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology 309, 767-779. |
 Enoka R.M. (2008) Comments on Point:Counterpoint: Spectral properties of the surface EMG can characterize/do not provide information about motor unit recruitment strategies and muscle fiber type. Journal of Applied Physiology 105, 1676. |
 Fan P., Yang Z., Wang T., Li J., Kim Y., Kim S. (2024) Neuromuscular control strategies in basketball shooting: Distance-dependent analysis of muscle synergies. Journal of Sports Science and Medicine 23, 571-580. |
 Farina D., Merletti R., Enoka R.M. (2014) The extraction of neural strategies from the surface EMG: An update. Journal of Applied Physiology 117, 1215-1230. |
 Fatela P., Mendonca G.V., Veloso A.P., Avela J., Mil-Homens P. (2019) Blood flow restriction alters motor unit behavior during resistance exercise. International Journal of Sports Medicine 40, 555-562. |
 Fatela P., Reis J.F., Mendonca G.V., Avela J., Mil-Homens P. (2016) Acute effects of exercise under different levels of blood-flow restriction on muscle activation and fatigue. European Journal of Applied Physiology 116, 985-995. |
 Goudriaan M., Papageorgiou E., Shuman B.R., Steele K.M., Dominici N., Van Campenhout A., Ortibus E., Molenaers G., Desloovere K. (2022) Muscle synergy structure and gait patterns in children with spastic cerebral palsy. Developmental Medicine & Child Neurology 64, 462-468. |
 Guo W., Kim Y., Wang J., Dong T., Tang X., Kim S. (2025) 60-second static stretching of lower limb muscles disrupts muscular performance and control in active male adults. Journal of Sports Science and Medicine 24, 195-204. |
 Healy R., Kenny I.C., Harrison A.J. (2018) Reactive strength index: A poor indicator of reactive strength?. International Journal of Sports Physiology and Performance 13, 802-809. |
 Jagim A.R., Schuler J., Szymanski E., Khurelbaatar C., Carpenter M., Fields J.B., Jones M.T. (2024) Acute responses of low-load resistance exercise with blood flow restriction. Journal of Functional Morphology and Kinesiology 9. |
 Kraemer W.J., Ratamess N.A. (2004) Fundamentals of resistance training: Progression and exercise prescription. Medicine & Science in Sports & Exercise 36, 674-688. |
 Loenneke J.P., Fahs C.A., Wilson J.M., Bemben M.G. (2011) Blood flow restriction: The metabolite/volume threshold theory. Medical Hypotheses 77, 748-752. |
 Loenneke J.P., Kim D., Fahs C.A., Thiebaud R.S., Abe T., Larson R.D., Bemben D.A., Bemben M.G. (2015) Effects of exercise with and without different degrees of blood flow restriction on torque and muscle activation. Muscle & Nerve 51, 713-721. |
 Lorenz D.S., Bailey L., Wilk K.E., Mangine R.E., Head P., Grindstaff T.L., Morrison S. (2021) Blood flow restriction training. Journal of Athletic Training 56, 937-944. |
 Matsunaga N., Aoki K., Kaneoka K. (2021) Comparison of modular control during side cutting before and after fatigue. Applied Bionics and Biomechanics. |
 McKay A. K. A., Stellingwerff T., Smith E. S., Martin D. T., Mujika I., Goosey-Tolfrey V. L., Sheppard J., Burke L. M. (2022) Defining Training and Performance Caliber: A Participant Classification Framework. International Journal of Sports Physiology and Performance 17, 317-331. |
 Oláh V., Třebický V., Maleček J., Michalička V., Wąsik J., Vágner M. (2025) Is countermovement jump height and one repetition maximum back squat associated with the peak force of a front kick with and without carried load?. Journal of Strength and Conditioning Research. |
 Olmos A.A., Montgomery T.R., Sears K.N., Roth B.L., Richardson L.D., Dinyer-McNeely T.K., Hammer S.M., Bergstrom H.C., Hill E.C., Succi P.J., Lubiak S., Trevino M.A. (2024) Blood flow restriction increases motor unit firing rates and input excitation of the biceps brachii during a moderate-load muscle action. Journal of Sports Sciences 42, 1891-1903. |
 Pandy M.G., Zajac F.E. (1991) Optimal muscular coordination strategies for jumping. Journal of Biomechanics 24, 1-10. |
 Patterson S.D., Hughes L., Warmington S., Burr J., Scott B.R., Owens J., Abe T., Nielsen J.L., Libardi C.A., Laurentino G., Neto G.R., Brandner C., Martin-Hernandez J., Loenneke J. (2019) Blood flow restriction exercise: Considerations of methodology, application, and safety. Frontiers in Physiology 10, 533. |
 Ramirez-Campillo R., García-Hermoso A., Moran J., Chaabene H., Negra Y., Scanlan A.T. (2022) The effects of plyometric jump training on physical fitness attributes in basketball players: A meta-analysis. Journal of Sport and Health Science 11, 656-670. |
 Saito A., Tomita A., Ando R., Watanabe K., Akima H. (2018) Muscle synergies are consistent across level and uphill treadmill running. Scientific Reports 8, 5979. |
 Steele K.M., Tresch M.C., Perreault E.J. (2013) The number and choice of muscles impact the results of muscle synergy analyses. Frontiers in Computational Neuroscience 7, 105. |
 Sun D., Yang T. (2023) Semi-squat exercises with varying levels of arterial occlusion pressure during blood flow restriction training induce a post-activation performance enhancement and improve vertical height jump in female football players. Journal of Sports Science and Medicine 22, 212-225. |
 Takarada Y., Takazawa H., Ishii N. (2000) Applications of vascular occlusion diminish disuse atrophy of knee extensor muscles. Medicine & Science in Sports & Exercise 32, 2035-2039. |
 Ting L.H., Chiel H.J., Trumbower R.D., Allen J.L., McKay J.L., Hackney M.E., Kesar T.M. (2015) Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 86, 38-54. |
 Turpin N., Guével A., Durand S., Hug F. (2011) Fatigue-related adaptations in muscle coordination during a cyclic exercise in humans. The Journal of Experimental Biology 214, 3305-314. |
 Turpin N.A., Uriac S., Dalleau G. (2021) How to improve the muscle synergy analysis methodology?. European Journal of Applied Physiology 121, 1009-1025. |
 Vigotsky A.D., Halperin I., Lehman G.J., Trajano G.S., Vieira T.M. (2017) Interpreting signal amplitudes in surface electromyography studies in sport and rehabilitation sciences. Frontiers in Physiology 8, 985. |
 Wang J., Fu H., QiangZhang Zhang M., Fan Y. (2022) Effect of leg half-squat training with blood flow restriction under different external loads on strength and vertical jumping performance in well-trained volleyball players. Dose Response 20, 15593258221123673. |
 Wharemate, J. (2021) Blood flow restriction as a method to elicit post
activation potentiation in trained female athletes. The University
of Waikato. |
 Williamson J.W., Crandall C.G., Potts J.T., Raven P.B. (1994) Blood pressure responses to dynamic exercise with lower-body positive pressure. Medicine & Science in Sports & Exercise 26, 701-708. |
 Wortman R.J., Brown S.M., Savage-Elliott I., Finley Z.J., Mulcahey M.K. (2021) Blood flow restriction training for athletes: A systematic review. American Journal of Sports Medicine 49, 1938-1944. |
 Xie P., Chang Q., Zhang Y., Dong X., Yu J., Chen X. (2022) Estimation of time-frequency muscle synergy in wrist movements. Entropy (Basel) 24. |
 Xu Y., Yang Y., He S., Yang C., Zhang S., Fu W., Li L. (2025) Running-induced fatigue influences lower extremity muscle synergy and related biomechanics. Gait & Posture 119, 163-170. |
 Yasuda T., Fujita S., Ogasawara R., Sato Y., Abe T. (2010) Effects of low-intensity bench press training with restricted arm muscle blood flow on chest muscle hypertrophy: A pilot study. Clinical Physiology and Functional Imaging 30, 338-343. |
 Yasuda T., Loenneke J.P., Thiebaud R.S., Abe T. (2015) Effects of detraining after blood flow-restricted low-intensity concentric or eccentric training on muscle size and strength. Journal of Physiological Sciences 65, 139-144. |
 Bao Nguyen, T., Yano, S. and Kondo, T. (2017) Muscle synergy analysis
in dart throwing. Annual International Conference of the IEEE
Engineering in Medicine and Biology Society 2534-2537.
|
|