|
NEUROMUSCULAR CONTROL IN LUMBAR DISORDERS*
*Doctoral
dissertation presented on the 6th of November
2003 at the the Faculty of Medicine of the University of Kuopio,
Finland, by permission of the Faculty of Medicine of the University
of Kuopio, Finland.
|
Departments of Physical and Rehabilitation Medicine,
Physiology, Neurosurgery, ClinicalNeurophysiology, University of Kuopio,
Kuopio, Finland
| Published
(Online) |
|
01
March 2004 |
©
Journal of Sports Science and Medicine (2004) 3, Suppl.4,
1 - 31
Search
Google Scholar for Citing Articles
This
review is based on the following orginal publications, which will be referred
to in the text as Studies 1-5:
1.
Leinonen, V., Kankaanpää, M., Hänninen, O., Airaksinen, O. and Taimela,
S. (2002) Paraspinal muscle responses during sudden upper limb loading.
European Journal of Applied Physiology 88, 42-49.
2.
Leinonen, V., Kankaanpää, M., Luukkonen, M., Hänninen, O., Airaksinen,
O. and Taimela, S. (2001) Disc herniation-related back pain impairs feed-forward
control of paraspinal muscles. Spine 26, E367-372.
3.
Leinonen, V., Kankaanpää, M., Luukkonen, M., Kansanen, M., Hänninen, O,
Airaksinen, O. and Taimela, S. (2003) Lumbar paraspinal muscle function,
perception of lumbar position and postural control in disc herniation-related
back pain. Spine 28, 842-848.
4.
Leinonen, V., Määttä, S., Taimela, S., Herno, A., Kankaanpää, M., Partanen,
J., Kansanen, M., Hänninen, O. and Airaksinen, O. (2002) Impaired lumbar
position sense in association with postural stability and motor and somatosensory
evoked potential findings in lumbar spinal stenosis. Spine 27,
975-983.
5.
Leinonen, V., Määttä, S., Taimela, S., Herno, A., Kankaanpää, M., Partanen,
J., Hänninen, O. and Airaksinen, O. (2003) Paraspinal muscle denervation,
paradoxically good lumbar endurance and abnormal flexion-extension cycle
in lumbar spinal stenosis. Spine 28, 324-331.
| ABSTRACT |
|
Impaired
motor and sensory functions have been associated with low back pain
(LBP). This includes disturbances in a wide range of sensorimotor
control e.g. sensory dysfunctions, impaired postural responses and
psychomotor control. However, the physiological mechanisms, clinical
relevance and characteristics of these findings in different spinal
pathologies require further clarification.
The purposes of this study were to investigate postural control,
lumbar muscle function, movement perception and associations between
these findings in healthy volunteers (n=35), patients with lumbar
disc herniation (n=20) and lumbar spinal stenosis (LSS, n=26).
Paraspinal muscle responses for sudden upper limb loading and muscle
activation during flexion-extension movement and the lumbar endurance
test were measured by surface electromyography (EMG). Postural stability
was measured on a force platform during two- and one-footed standing.
Lumbar movement perception was assessed in a motorised trunk rotation
unit in the seated position. In addition, measurements of motor-(MEP)
and somatosensory evoked potentials (SEP) and needle EMG examination
of lumbar multifidus muscles were performed in the LSS patients.
Clinical and questionnaire data were also recorded.
A short latency paraspinal muscle response (~50 ms) for sudden upper
limb loading was observed. The latency of the response was shortened
by expectation (p=0.017). The response latency for unexpected loading
was similar in healthy persons and disc herniation patients but
the latency was not shortened by expectation in the patients (p
= 0.014). Also impaired postural control (p < 0.05) and lumbar
movement perception (p = 0.012) were observed in disc herniation
patients. The impaired lumbar movement perception (p=0.054) and
anticipatory muscle activation (p = 0.043) tended to be restored
after successful surgery but postural control had still not recovered
after 3 months of follow-up. The majority of LSS patients were unable
to sense a rotational movement in the lumbar area and thus had clearly
impaired lumbar movement perception (p = 0.006). Abnormal MEPs had
only inconsistent and SEPs showed no associations with impaired
movement perception and postural stability in LSS. Abnormal needle
EMG findings and flexion-extension activation of paraspinal muscles
were frequently observed in LSS patients. Lumbar paraspinal muscle
endurance was better than in previously evaluated healthy subjects
and chronic LBP patients (p < 0.001).
The results demonstrated clearly impaired lumbar sensory and motor
function in sciatica and LSS patients. The pure reflex activation
of paraspinal muscles was not affected in sciatica but a difference
was found in the premotoneuronal response control. The impaired
proprioceptive functions and premotoneuronal response control seem
to recover at least partially but the maintenance of postural stability
is a complex activity which does not seem to recover automatically
in operated sciatica patients at least in three months follow-up.
Paraspinal muscle denervation and dysfunction were clearly detectable
in LSS but lumbar paraspinal muscle endurance was unexpectedly good.
KEY
WORDS: Low back pain, intervertebral disk displacement, spinal
stenosis, electromyography, evoked potentials, posture, psychomotor
performance, comparative study, prospective study
|
| INTRODUCTION |
|
Low
back pain (LBP) is the most common musculo-skeletal disorder causing
huge humanitarian and economical costs (Andersson, 1999;
Aromaa et al., 2002).
The pain may arise from injured structures within the lumbar spine,
however the anatomical cause and functional consequences of the
LBP often remain undefined. In chronic pain, the precise aetiology
is even less well understood. Imaging studies such as MRI visualise
the macroscopic structural changes but similar changes are often
found in healthy subjects and frequently the correlation with the
clinical condition seems to be rather low (Jensen et al., 1994;
Jarvik et al., 2001).
The chronic LBP is a multidimensional problem including pain and
functional disability with its associated socioeconomical consequences.
Impairments in neuromuscular control have often been associated
with chronic LBP and are considered a probable link between pain
and disability (Luoto, 1999;
Ebenbichler et al., 2001;
Holm et al., 2002;
Hodges and Moseley, 2003;
van Dieen et al., 2003b).
There is increasing evidence that the impaired functions can recover
with treatment and be restored by active rehabilitation (Ebenbichler
et al., 2001).
The appropriate muscular control and movement perception is of vital
importance in preventing low back injury. The protection against
injury requires anticipation of events and adequate muscular responses.
Both abnormal and missing protective reflexes could possibly lead
to trauma or microtrauma of muscles, nerves, intervertebral discs
and ligamentous spine during loading (McGill, 1997).
The impaired neuromuscular control becomes particularly emphasised
in conditions of chronic pain, which are also the most expensive
for the society and the most demanding for the health care system.
The etiological research is important for developing more effective
treatment modalities especially in prolonged and chronic pain conditions.
Unfortunately we have only a limited understanding of the time-course,
appearance and clinical relevance of changes in motor control in
LBP attributable to different pathophysiological mechanisms.
This study has investigated the postural control and lumbar motor
and proprioceptive functions in specific lumbar disorders of disc
herniation and lumbar spinal stenosis. The purpose was to evaluate
the phenomena involved in motor control in LBP and patient groups
with specific back disorders were used as experimental models.
|
| GENERAL
OVERVIEW |
WHO
has developed the International Classification of Functioning, Disability
and Health (ICF) as a framework for the description of health and
health-related states (WHO, 2001).
Functioning encompasses all body functions, activities and participation,
and disability includes impairments, activity limitations and participation
restrictions. These functions interact with environmental and personal
factors defining the health condition (Figure
1). This taxonomy model can be used in the creation of assessment
methods of lumbar disorders (WHO, 2001).
The current study is focused on "Sensory functions and pain"
(b2) and "Neuromusculoskeletal and movement-related functions"
(b7).
Pain is defined as an unpleasant sensation and emotional experience
associated with actual or potential tissue damage, or described in
terms of such damage (IASP, 1979,
Merskey and Bogduk, 1994).
The acute pain usually eases during tissue healing or after the impending
threat of tissue damage has passed. In conditions of chronic neuropathic
pain, the sensory processing of the affected body region becomes abnormal
(Watkins and Maier, 2002)
and there may become detectable changes in central information processing
(Peyron et al., 2000;
Juottonen et al., 2002;
Farina et al., 2003)
and altered pain experience.
Low back pain (LBP) is defined as pain in the lumbar area. It is a
common problem which affects the majority of the population. The lifetime
prevalence of LBP varies from 60 to 90 percent with an annual incidence
of 5% (Frymoyer, 1988;
Aromaa et al., 2002).
In the majority of cases, the back problems tend to show the first
symptoms before the age of twenty (Leboef-Yde and Kyvik, 1998).
Usually the pain is acute and heals by itself in less than two months,
but most of these cases will experience relapses with each episode
becoming worse and worse. Approximately 5 to 10% of cases become chronic,
lasting over two months and creating a major medical challenge (Frymoyer,
1988).
Despite the progress in diagnostic methods, the aetiology of back
pain remains uncertain in most of the cases. It has been estimated
that a precise diagnosis can be determined for less than 20% of low
back pain patients (White and Gordon, 1982,
Nachemson, 1985,
Deyo and Weinstein, 2001),
depending partly on the interpretation of radiographic findings. Furthermore,
the radiological and clinical findings and symptoms do not invariably
correlate. As many as half of the asymptomatic subjects exhibited
abnormal changes in their discs according to the MRI study by Jensen
et al., (1994)
and the MRI findings alone have only limited importance (Videman et
al., 2003).
Nerve-root symptoms occur only in one percent of acute LBP patients,
but they are often associated with enduring and persistent pain. Sciatica
is defined as pain in a distribution area of a lumbar nerve root,
often accompanied by sensory and motor deficits. Sciatic pain requires
that there must be mechanical and inflammatory stimuli to lumbar nerve
roots. The most common cause of sciatica is a herniated lumbar disc
but in the ageing population, there is now an increased prevalence
of spinal stenosis as a cause of sciatica (Frymoyer, 1988).
This study defines the LBP syndrome as a multidimensional problem
including pain, functional disability and socioeconomical consequences.
The low back syndrome seems to be a continuous process, and the functional
disorders vary with respect to the stage and duration of the illness
and physical and psychological stress. However, the time course of
the LBP and the relation between time and symptoms are poorly understood.
According to experimental studies, the spatial or temporal overloading
of spinal structures leads to micro-injuries, inflammation, pain and
neuromuscular dysfunction (Claude et al., 2003;
Solomonow et al., 2003a).
This dysfunction has been claimed to be associated with clinical pain
(Indahl, 1999;
Solomonow et al., 2003b).
Although in chronic pain, the psychosocial component often seems to
be important, the origin of the syndrome is organic and thus an evaluation
of spinal function is of clear value.
|
| REVIEW
OF THE LITERATURE |
| Lumbar
disorders
Classification
of lumbar disorders
Classification of the LBP is still a challenging problem. Lumbar
diseases are often divided into specific and non-specific conditions.
In non-specific (idiopathic) cases the origin of the pain is unknown,
however, there are several potential sources of pain like muscles,
tendons, ligaments, nerves, discs etc. The (rare) specific spinal
diseases are attributable to fractures, tumours and infections etc.
The more common specific conditions are disc herniation and lumbar
spinal stenosis, which are the main causes of sciatica (Frymoyer,
1988;
Deyo and Weinstein, 2001).
The LBP is also divided by its duration, usually the pain lasting
less than 6 weeks is called acute, 6-12 weeks is subacute and over
3 months is chronic LBP (Bigos et al., 1994).
Low back pain
Pathophysiology
The underlying mechanisms of LBP are usually unknown but there are
several potential sources and causes of pain. Currently, there seems
to be only concepts but little hard evidence for the development
of the LBP. One potential course of the syndrome could be as follows:
first the micro-injury e.g. to the muscles, tendons, ligaments,
intervertebral disc or endplate. Injury is followed by macrophage
and neutrophil accumulation and inflammation (Ahn et al., 2002;
Burke et al., 2002).
Static lumbar flexion has been indicated to cause the development
of creep and micro-injuries of the collageneous structures in the
lumbar ligaments. This elicits acute inflammation and hyperexcitability
of the multifidus muscle (Claude et al., 2003;
Solomonow et al., 2003a).
This reflexive muscular stiffness is thought to protect the injured
structures and enabling tissue healing (Claude et al., 2003;
van Dieen et al., 2003a;
Solomonow et al., 2003a).
Reactive oxygen species (ROS) seem to contribute to muscle fatigue
(Reid, 2000)
and therefore they may have role also in the pathophysiology of
LBP. Dorsal ramus neuropathy is thought to be one potential cause
of LBP (Sihvonen, 1995).
Experimental studies have indicated that pain arising from the passive
or active structures can lead to pathological activation of the
paraspinal muscles at the same and adjacent segments (Indahl, 1999;
Solomonow et al., 2003a).
Despite this important finding, one still requires unequivocal clinical
evidence about these protective reflexes. In the clinical course
of a long-lasting spinal disorder, ultimately, the chronic pain
may lead to deconditioning of the muscles and neural modulation
of the central nervous system (Flor et al., 1997;
Kankaanpää, 1999;
Luoto, 1999).
Symptoms and findings
The primary diagnostic evaluation includes perusal of the medical
history and a physical examination. This aims to exclude or diagnose
serious causes of pain and nerve root compression. In addition,
the social and psychological factors causing distress possibly complicating
the recovery should be clarified (Bigos et al., 1994;
Waddell et al., 1996;
Deyo and Weinstein, 2001).
Patients suffering prolonged pain and when there is suspicion of
a serious illness or persisting radicular symptoms need further
evaluation.
In the acute condition, imaging is often unnecessary (Deyo and Weinstein,
2001). The plain
radiograph is currently the basic examination procedure for prolonged
LBP lasting over six weeks (Jarvik and Deyo, 2002).
The primary supplementary examination is MRI, which reveals also
macroscopic soft tissue changes (Herzog et al., 1995)
and can even be considered as the primary procedure in the patients
with radicular symptoms when surgery is potentially indicated. Disc
degeneration is a usual finding also in asymptomatic subjects (Boden
et al., 1990;
Tertti et al., 1991;
Jensen et al., 1994;
Jarvik et al., 2001)
and does not appear to have a cause-effect relationship with pain.
Disc disruptions seems to have clinical significance (Kuslich et
al., 1991; Moneta
et al., 1994),
but the clinical relevance of the indicative MRI findings of the
disc disruptions is rather weak (Videman et al., 2003)
and the need for their invasive evaluation (discography) and treatment
is not clear. Therefore, evaluation of the functional ability is
often more important than anatomic findings (Bigos et al., 1994;
Waddell et al., 1996;
Deyo and Weinstein, 2001).
Treatment and prognosis
LBP is not a self-limiting condition (Hestbaek et al., 2003).
Effective pain medication is essential in the acute condition, and
it may prevent the pain from becoming chronic (Bigos et al., 1994;
Waddell, 1996;
van Tulder et al., 1997).
It is important to inform the patient about the benign nature of
the syndrome, the need to avoid bed rest and to encourage the patient
to maintain normal daily activities (Malmivaara et al., 1995).
In acute benign pain it is crucial that the diagnosis and interpretation
of the radiological findings are undertaken to ensure that the patients
do not progress to illness behaviour and fear-avoidance (Koes et
al., 2001), which
are often complicating factors promoting chronic LBP and disability.
In prolonged pain, activating procedures are recommended (van Tulder,
1997), and in
the case of radiating pain surgeon should be consulted at the latest
after six weeks of observation (Deyo and Weinstein, 2001).
The patient should be encouraged to exercise and make active use
of the back. Spinal manipulation and physical therapy are potential
alternatives to treatment (Deyo and Weinstein, 2001).
If not started before, then an active rehabilitation process including
guided and self-motivated exercises should be added to the treatment
of pain lasting over six weeks. In chronic pain, a comprehensive
evaluation of the condition and an intensive integrated rehabilitation
should be undertaken (Bigos et al., 1994;
Guzman et al., 2002).
The good prognosis of the acute pain should be emphasised from the
beginning but also the patient should be informed about the frequent
recurrence of the pain. Only five to 10 percent of the cases become
chronic, lasting over three months but those cases result in the
most severe medical and economical hardship.
Disc herniation
Pathophysiology
In the herniated disc, the nucleus pulposus has gone through the
ruptured annulus fibrosus. Disc herniation causes mechanical compression
of the nerve roots and chemical irritation mainly by activation
of the inflammatory processes (Kuslich et al., 1991,
Olmarker et al., 1993).
The tumor necrosis factor-alpha (Olmarker and Larsson, 1998,
Olmarker and Rydevik, 2001)
and several other cytokines (Watkins and Maier, 2002)
appear to be clearly associated with the inflammatory process in
sciatica. The use of specific anti-TNF- treatment may alter the
treatment strategy of acute sciatica in the future (Watkins and
Maier, 2002;
Karppinen et al., 2003).
Symptoms and findings
The disc rupture itself can be painful and, in addition to the local
LBP, they can also mimic the radicular symptoms evoked by a herniated
disc (Karppinen et al., 2001).
The radicular symptoms include pain, numbness, sensory disturbances,
paresthesia and motor weakness. In severe cases (cauda equina syndrome)
urinary retention, anal incontinence or sensory loss in a saddle
distribution may be present (Frymoyer, 1988).
The disc herniation is not visible in the plain radiograph but can
be detected by MRI (Herzog
et al., 1995)
or optionally by CT.
Treatment and prognosis
The radicular symptoms usually disappear in three months by conservative
treatment. In those cases with persistant severe symptoms over six
weeks with a clear radiological finding, surgery is usually considered.
Intolerable pain, cauda equina syndrome or acute paresis/paralysis
may require emergency surgery (Kostuik et al., 1986;
Bigos et al., 1994).
Due to the good spontaneous recovery, only approximately ten percent
of the patients need to undergo surgery (Frymoyer, 1988),
however, the result of surgery is better if it is initiated within
three months from the beginning of the symptoms (Hurme and Alaranta,
1987). One year
after surgery 50-85% of the operated patients are free of sciatica
(Hoffman et al., 1993;
Junge et al., 1995).
However, there is a considerable recurrence of the syndrome. The
need for re-operation may be up to 20% during 13 years follow-up
(Nykvist et al., 1995),
according to Finnish Hospital Discharge Register 14% of patients
needed re-operation during a 10-year follow-up (Österman et al.,
2003).
According to an early randomised trial comparing surgery and conservative
treatment, the surgery was more effective for two years follow-up,
but from two until ten years the results were statistically equivalent
(Weber, 1983),
though a later reanalysis has found a slight benefit favouring surgery
also during a longer follow-up. The recent RCT showed faster recovery
after surgery but only minor differences between conservative care
and surgery at two-year follow-up (Österman et al., 2002).
The current conservative treatment includes pain medication and
patient information. Bed rest should be avoided and the patient
should be encouraged to resume normal daily activities (within the
limits imposed by the pain) as in non-specific LBP. New promising
treatments, such as therapy with TNF-alpha antibodies, are also
on the horizon (Karppinen et al., 2003).
Lumbar spinal stenosis
LSS is a notable degenerative disorder of the lumbar spine, responsible
for low back and lower extremity pain. The symptoms of the disorder
arise from compression of the cauda equina due to degenerative processes
leading to anatomical stenosis of the lumbar spinal canal and intervertebral
foramina (Verbiest, 1954).
Pathophysiology
The anatomical narrowness of spinal canal is the basic aetiology
for LSS but the syndrome is caused by degeneration of lumbar spine
leading to facet arthrosis and disc degeneration with osteophytes
and thickening and calcification of ligamentum flavum (Rauschning,
1993; Fritz
et al., 1998;
Spivak, 1998).
The pathophysiology of the symptoms is poorly known, but it is thought
that the mechanical compression irritates the nerve roots and causes
the symptoms of the disease. However, the symptoms do not clearly
correlate with the degree of the stenosis, and there is a considerable
fluctuation in the symptoms (Amundsen et al., 1995;
Jönsson et al., 1997).
One potential explanation is vascular compression theory based on
the venous congestion caused by the mechanical compression of the
cauda equina veins, leading to decreased blood flow and subsequent
dysfunction of the lumbar nerve roots (Porter, 2000).
Recent experimental studies have supported that theory by indicating
ectopic firing caused by venous stasis of lumbar nerve roots and
inhibition of ectopic firing after decompression (Ikawa et al.,
2003).
Symptoms and findings
The symptoms include LBP, intermittent claudication and radicular
symptoms such as pain, numbness, weakness and loss of sensation
in the lower limbs. Claudication and radicular symptoms are often
provocated by spine extension and revealed by flexion and the claudication
eases slowly, when the subject stops walking (Spivak, 1998).
The plain radiograph can indicate LSS but the diagnosis is based
on MRI or CT findings correlating with the symptoms (Katz et al.,
1994).
Treatment and prognosis
After diagnosis of LSS, the options are follow-up, conservative
treatment or surgery. The mild symptoms are usually treated conservatively,
but severe stenosis often requires decompressive surgery. Conservative
treatment includes analgesic medication, physical therapy, spinal
support and calcitonin therapy (Eskola et al., 1992),
however, there is still need for the RCT based evidence on the effect
of conservative treatment modalities. If the symptoms and disability
are intolerable despite conservative treatment, then surgery is
indicated. The symptoms are eased, and the extent of disability
decreased after surgery (Atlas et al., 2000),
but the long-term outcome shows large variation (Herno, 1995;
Hurri et al., 1998).
The natural course of LSS is unclear but according to a recent RCT,
surgery seems to be better than conservative care in moderately
severe LSS at one-year follow-up (Slätis et al., 2002).
Motor control
Classification of motor control to voluntary and automatic actions
Human motor control includes schematically three different co-operating
levels (Figure 2). The highest
level is planning of the movement, the lowest level is spinal reflexes,
and the intermediate level connects the highest and lowest levels
for the execution of the task (Kalaska and Drew, 1993).
The terms voluntary and reflex are widely used in scientific papers
and in everyday conversation, but the exact meaning of these terms
is not clear and need some definition (Prochazka et al., 2000).
This study defines focused attention encompassing conscious (voluntary)
control and subsidiary activation encompassing automatic motor programs
(including reflexes). Isolated voluntary and automatic actions are
rarely seen in real life, the normal functions are combinations
of different levels of control. Reflexes assist in voluntary functions
and cortical activation may regulate reflexes. The primary motor
cortex is involved in reflex inhibition via the descending pathways,
which vary their activity level according to the attention, arousal
and emotional state of the subject.
Focused attention
Voluntary movements are programmed in the brain. Several cortical
areas e.g. supplementary motor cortex, basal ganglia, cerebellum,
thalamus, anterior gyrus cinguli and primary and supplementary somatosensory
cortex are linked to the primary motor cortex. These centres are
involved in the planning of the movement. The final command to perform
the motion is initiated by the pyramidal cells of primary motor
cortex and transmitted through the corticospinal pathway to the
ventral horn of the spinal cord. At a specific spinal level, the
upper motor neuron synapses with the alpha motor neuron which then
transmits the command to muscle fibers of its own motor unit (Rothwell,
1994).
Subsidiary movement
The subsidiary muscle activations are often associated with voluntary
movements and are function of servo actions needed e.g. in postural
control (Rothwell, 1994).
These actions include anticipatory postural adjustments and are
usually automatic i.e. unconsciously controlled but are more complex
than simple reflexes. The responsible level controlling these fast
actions is intermediate, also called the extrapyramidal system.
In addition to thalamus and basal nerve nuclei, this involve the
cerebellum and basal ganglia, which are involved in the tuning of
the fine movements, coordination, timing, motor learning and muscle
tone.
Automatic motor programs
Reflexes are the fastest way to control movements. They are categorically
divided into mono-, di- and polysynaptic reflexes. The monosynaptic
reflex is the simplest and fastest of the three reflex types. The
electrical reflex latency varies from ~10 ms in spinal muscles to
~50 ms in distal lower limb muscles depending on the distance of
respective muscle from the spinal cord or cranial nerve nuclei.
The latency consists of the sensory impulse conduction in sensory
neurons, synaptic transmission and motor impulse conduction in the
motor neurons (Rothwell, 1994;
Schmidt and Lee, 1999).
In addition, there is a notable time lag from the electrical activation
of the muscle cells to the mechanical force produced by the muscle
(Rothwell, 1994;
Schmidt and Lee, 1999).
In complex responses, the relative time for information processing
is longer.
In the simple monosynaptic stretch reflex, muscle spindles sense
the stretching of the muscle fibres. The information is mediated
by the Ia sensory afferent nerves into the dorsal horn at the respective
level of the spinal cord. These sensory nerve cells synapse with
the motor neurons activating the corresponding muscle. In more complex
reflexes, an increasing number of interneurons are involved in mediating
the reflex (Rothwell, 1994).
The muscle activation is modulated also by the gamma motor neuron
system. These types of reflexes are usuall involved in voluntary
and subsidiary movements e.g. permitting the fluency of movements
by controlling the agonist-antagonist muscle activation.
Postural control
The equilibrium of the body is essential for locomotion and performing
other types of limb movements. The purpose of the postural control
system is to support, stabilize and balance with the aim of maintaining
postural stability. Postural control functions at three tightly
connected levels i.e. postural reflexes, triggered reactions and
voluntary movements these being listed from fastest to slowest level
of action and from the lowest to highest level of cognition, respectively.
The somatosensory, vestibular and visual receptors provide the sensory
information needed in this process (Rothwell, 1994).
The sensory information is transmitted by the ascending pathways
of the spinal cord and the motor commands are transmitted by the
descending pathways which synapse with the lower motor neurons.
The major descending pathways controlling body posture are the vestibulospinal
tracts. The neurons of the vestibulospinal tracts synapse with the
same alpha motor neuron as the corticospinal tract (Rothwell, 1994).
Postural responses are modulated by feedback information but also
pre-programmed functions are needed (Schmidt and Lee, 1999;
Hodges, 2003).
The cerebellum is important in maintaining the balance and adaptation
and learning of postural reflexes and feed-forward responses (Horak
and Diener, 1994;
Rothwell, 1994).
Postural control is a complex procedure and vulnerable to disruption
by a wide variety of disorders (Horak and Nashner, 1986;
Rothwell, 1994).
In addition to the neurological and vestibular diseases, postural
control plays also a significant role in several musculo-skeletal
impairments including back and joint disorders (Alaranta et al.,
1994). Increasing
age starts to have detrimental effects on postural control after
the fifth decade of life (Alaranta et al., 1994;
Aalto, 1997).
Motor control of the lumbar spine
Lumbar spinal anatomy has been described in detail by Bogduk and
Twomey (1991)
and reviewed in the association with LBP by Indahl (1999)
and Kankaanpää (1999).
According to Panjabi (1992),
the spinal stability system consists of three subsystems, which
are the passive spinal column, the active spinal muscles and the
neural control unit. An overloading or dysfunction of any of these
subsystems may lead to injury if there is a failure of compensation
mechanisms (Panjabi, 1992).
Intra-abdominal pressure, diaphragm, pelvic floor and abdominal
muscle activation have been shown to have a significant role in
spinal control (Hodges, 2003).
The simple stability and instability concept in the low back syndrome
has recently been challenged and the importance of neural control
system has been emphasised. Hodges (2003)
has shown that CNS does not simply stiffen the spine and restrict
the spinal motion, but actively uses movements to maintain equilibrium
in the posture. Lund (2003)
has indicated that mechanical LBP is associated with restricted
but painful motion rather than mechanical instability. This indicates
that a potential pathomechanism of "the instability" is
dysfunction of neuromuscular control system.
Segmental function of the lumbar spine
Two vertebrae, their intervertebral disc and facet joints form the
functional spinal unit (FSU, Pope et al., 1993).
The ligamento-muscular reflex between multifidus muscles, zygapophysial
joints and intervertebral ligaments has been demonstrated in the
control of the intricate neuromuscular balance in the lumbar motion
segment (Solomonow et al., 1998;
Indahl, 1999).
The possible interactive responses between injured or diseased spinal
structures, i.e., disc or facet joints, and the paraspinal musculature
may lead to segmental dysfunction of the lumbar spine (Sihvonen
et al., 1991;
Indahl et al., 1995;
1997; Kaigle
et al., 1998).
Kinetic chain
Thoracolumbar fascia connects the spine, upper limbs and pelvis
and via the sacrotuberous ligament the pelvis to the lower limbs.
This allows dynamic load transfer from spine to legs (Vleeming et
al., 1995).
Paraspinal, gluteal and hamstring muscles exhibit sequential activation
during sagittal trunk flexion and extension (Paquet et al., 1994;
Leinonen et al., 2000).
Dysfunction of any part of the integrated system may lead to abnormal
load transfer between low back and pelvis. Therefore, it seems rational
that the hip extensor muscles, in addition to the spinal and abdominal
musculature, will be subjected to deconditioning in CLBP patients,
if the use of these muscles is avoided (Kankaanpää et al., 1998).
Feedback control
The appropriate proprioceptive information is essential for motor
control. In unpredictable postural perturbation, the control strategy
is based on sensory information transmitted from the receptive structures
and during intended movements, feedback information is needed for
error correction (Schmidt and Lee, 1999).
The proprioceptive information of the body and limb movements originate
from the muscle spindles, the Golgi tendon organs, joint and the
cutaneous receptors (Rothwell, 1994;
Schmidt and Lee, 1999).
Muscle receptors seem to play a major role in joint position sense
(McCloskey, 1978;
Pedersen et al., 1998).
The increased repositioning error after paraspinal muscle vibration
and impaired lumbar position sense after lumbar paraspinal muscle
fatigue emphasize the importance of muscle spindles in the positioning
of lumbosacral spine (Brumagne et al., 1999;
2000; Taimela
et al., 1999).
Tactile sensations and conscious proprioceptive information about
the body are mediated to the somatosensory cortex via the dorsal
columns, the fasciculus gracilis from lower limbs and the lower
part of the body and by the fasciculus cuneatus from the upper limbs
and the upper part of the body. The dorsal spinocerebellar and probably
also the spinothalamic tracts transmit the proprioceptive information
needed in the maintenance of postural stability (Rothwell, 1994).
The precise neurophysiological mechanisms processing the proprioceptive
input in conscious sensory perception remain to be determined but
probably involve coordinated inputs from cerebellum, thalamus and
somatosensory cortex.
Feed-forward control
In predictable postural perturbation, the CNS can plan control strategies
in advance. According to the recognition schema theory, muscle activation
can be pre-programmed based on initial condition, environmental
outcome and expected sensory consequences (Schmidt, 1975;
Schmidt and Lee, 1999).
Upper (Cordo and Nashner, 1982,
Zattara and Bouisset, 1988,
Hodges and Richardson, 1996)
or lower (Hodges and Richardson, 1998) limb voluntary movements
evoke non-conscious muscle activation in the trunk muscles via a
feed-forward mechanism. This refers to the activities of the central
movement control system, which maintains postural stability and
prepares the trunk to bear a potentially increasing load by activating
certain trunk muscles. These trunk muscles which maintain the dynamic
spine stability, are activated before the activation of the prime
muscles responsible for the gross limb movement without an afferent
input from the respective trunk movement (Belen'kii et al., 1967;
Cordo and Nashner, 1982;
Friedli et al., 1988;
Zattara and Bouisset, 1988;
Aruin and Latash, 1995;
Hodges et al., 1999).
Measurements of lumbar function
The clinical examination include inspection, palpation, range of
spinal motion, testing of lower limb sensory function, muscles and
reflexes. These examinations with the functional and provocation
tests form the basics of the evaluation of lumbar function in LBP
(Deyo and Weinstein, 2001).
The test results are usually non-specific and evaluated subjectively,
but their repeatability is acceptable and they are very useful,
especially when they are correlating with the subjective symptoms.
Neurophysiologic assessments include neurography, electromyographic
examination and motor and somatosensory evoked potential measurements.
They are used in the assessment of nerve injury and in the diagnosis
of the level of nerve injury (Dvorak et al., 2000).
Several functional measurements can be used in the study of LBP.
Surface electromyography measurements are used in the assessment
of muscle function (Sihvonen, 1995)
and endurance (Kankaanpää, 1999).
Muscle strength (Rantanen, 2001)
and lumbar kinematics are measured with a wide variety of methods
(Lund, 2003).
Postural control can be measured on a force-platform either with
or without simultaneous EMG and trunk motion measurements (Luoto,
1999). They have
been mainly used in assessing physiological and pathophysiological
mechanisms of lumbar function (Hodges, 2003).
The test results usually overlap with values obtained from back
healthy subjects. Therefore they are not of any great benefit in
the diagnosis but can be used for example in the planning of rehabilitation
procedures and in the objective evaluation of the effectiveness
of the rehabilitation. The diagnostic value of the functional measurement
is often rather poor and their clinical use is also hindered by
a lack of standardisation.
Pain and sensory-motor control
Pain and proprioception
Impaired lumbar proprioception is associated with LBP (Taimela et
al., 1999).
An increased repositioning error during trunk movement has been
observed in LBP patients (Gill and Callaghan, 1998;
Brumagne et al., 2000;
Newcomer et al., 2000).
Paraspinal muscle spindles seem to be important in the correct positioning
of the lumbosacral spine and the muscle spindle input may be decreased
in lumbar pain (Brumagne et al., 1999;
Taimela et al., 1999).
In addition, experimental muscle pain has been shown to affect the
central modulation of proprioceptive signals of jaw muscle spindles
(Capra and Ro, 2000).
However, there is experimental evidence that the nociceptive input
can actually enhance the central sensitivity to the mechanoreceptor
input (Torebjörk et al., 1992).
Pain and trunk muscle function
Pain has clear effects on motor control. LBP can induce changes
in neuromuscular control and motor performance. The function and
co-ordination of the muscles stabilising the lumbar spine seem to
be impaired in patients suffering from LBP (Hodges et al., 1996;
Wilder et al., 1996).
Delayed or absent trunk muscle activation has been observed in low
back pain patients during upper (Hodges and Richardson, 1996) and
lower limb movements (Hodges and Richardson, 1998)
and after experimental pain (Hodges et al., 2003).
A different muscle response pattern to sudden load release (Radebold
et al., 2000;
2001) and during
expected and unexpected upper limb and trunk loading (Wilder et
al., 1996; Magnusson
et al., 1996)
has been found in CLBP patients compared with healthy controls.
Psychosocial stress can change spinal muscle activation and loading.
However, notable individual differences have been observed in its
importance (Marras et al., 2000;
Davis et al., 2002).
CLBP patients have been claimed to exhibit an increased paraspinal
(Roy et al., 1989)
and gluteal (Kankaanpää et al., 1998)
muscle fatigability. During trunk flexion-extension, CLBP patients
had increased paraspinal muscle activity when acting as antagonist
(Ahern et al., 1988;
Sihvonen et al., 1991;
Sihvonen, 1995)
and decreased activity when acting as agonist (Sihvonen et al.,
1991), with
similar activation patterns being observed during experimental pain
(Zedka et al., 1999).
The CLBP and experimental muscle pain induced increased paraspinal
muscle activity in the normally silent period and decreased activity
in the normally active period of the gait (Arendt-Nielsen et al.,
1996).
LBP and general motor control
The impaired postural control is associated with chronic lumbar
disorders (Byl and Sinnot, 1991;
Luoto et al., 1996).
A slow psychomotor reaction time is also associated with back pain
(Taimela et al., 1993).
Poor postural and psychomotor control (Taimela, 1992)
is a known risk factor for accidental injuries, but in lumbar disorders
it is not yet known whether the poor postural control is a consequence
of pain or neuromuscular impairment or a potential major source
of the disorder. Thus, there appears to be little prospective evidence
on whether the impaired motor control is pre-existing (risk factor)
or a secondary phenomenon (consequence) of the musculoskeletal disorder.
The muscle dysfunction and deconditioning seem to be rather a consequence
of the tissue injury and pain. However, this claim needs further
prospective evaluation.
Pain seems to decrease the muscle activation amplitude during voluntary
contractions and increase the muscle activation during automatic
contractions (Zedka et al., 1999).
One potential explanation for this is the hypothesis based on the
model of fear-avoidance behaviour, where the decrease in central
output is revealed as decreased primary motor activation and decreased
inhibition of reflexes.
Active physical rehabilitation has been claimed to restore the impaired
neuromuscular functions (Wilder et al., 1996;
Kankaanpää et al., 1999).
However, impaired postural control failed to improve after rehabilitation
and after unsuccessful treatment the condition may even deteriorate
(Luoto et al., 1998). Despite the widespread acceptance of the importance
of stabilising functions of the spine, there is still a need of
evidence based on randomised controlled trials on the effect of
stabilising exercises in LBP.
In conclusion extensive variety of changes in motor and sensory
control and trunk muscle function have been reported. However, they
occurrence and clinical relevance in LBP with different pathophysiological
causes remains undefined. In addition, the time-course of the changes
and the effect of interventions are rarely evaluated.
|
| AIMS
OF THE STUDY |
The
purpose of this study was to evaluate involuntary motor control in
patients with specific lumbar disorders of disc herniation and lumbar
spinal stenosis in relation to healthy controls.
The specific aims
To investigate the latency and magnitude of the reflex responses in
paraspinal muscles after unexpected and expected upper limb loading
and to investigate the effect of anticipation and external spinal
support on these responses i) in healthy subjects, ii) in patients
with disc herniation before and after discectomy surgery.
To assess lumbar movement perception i) in healthy subjects, ii) in
patients with disc herniation before and after discectomy surgery,
iii) in patients with LSS.
To evaluate postural stability i) in healthy subjects, ii) in patients
with disc herniation before and after discectomy surgery, iii) in
patients with LSS.
To determine paraspinal muscle function, innervation and endurance
in patients with LSS.
|
| METHODS |
Subjects
Total of 81 subjects volunteered to participate in the study (Table
1). All subjects provided informed consent prior to their participation.
The study was approved by the Kuopio University Hospital Research
Ethics Board, and it was performed according to the Declaration of
Helsinki. The subjects had not had any previous spine surgery or cervical
radicular symptoms. Additional exclusion criteria were neurological,
metabolic or severe cardiovascular disease.
Healthy subjects
Thirty-five healthy subjects were recruited in the study (1-3).
Patients with herniated lumbar disc
The study included 20 patients selected for surgery due to lumbar
disc herniation (15 males and 5 females; study 2, 3). The patients
underwent microdiscectomy performed by a neurosurgeon (study 3). Baseline
measurements were done one day before the surgery and the follow-up
measurements 3 months after the surgery (study 2, 3).
Patients
with lumbar spinal stenosis
The study included 26 LSS patients (11 men, 15 women; study 4, 5).
The diagnosis was based on the patients' symptoms and signs and
was confirmed by computed tomography (CT) or magnetic resonance
imaging (MRI).
Assessment of paraspinal muscle function
Surface EMG
Bipolar surface electromyography (EMG) was recorded bilaterally
over the paraspinal muscles at T12-L1 and L5-S1 levels by a four
channel ME 3000P EMG system (Mega Electronics Ltd, Kuopio, Finland)
with disposable Ag/AgCl surface electrodes (Medicotest, Olstykke,
Denmark). The electrodes were placed on the erector spinae (ES,
T12-L1 level) and multifidus (MF, L5-S1 level) muscles as recommended
by Biedermann et al., (1991).
The raw EMG signal was recorded at the sampling rate of 2 kHz and
band-pass filtered between 7-500 Hz with an analogue filter, amplified
(differential amplifier, CMRR>110 dB, gain 1000, noise<1µV),
analogue-to-digital converted (12-bit), and stored in a personal
computer for later analysis. (study 1-3, 5).
Paraspinal
muscle responses for sudden upper limb loading
Back muscle reaction time for unexpected (eyes closed) and expected
(eyes open) upper limb loading the procedure, the subject was standing
and holding a box in the hands while a weight of 0.68 kg was suddenly
dropped from the height of the subject's eyes into the box equipped
with a marker switch indicating the moment of impact. Twelve consecutive
measurements were performed in sequences of three trials with the
eyes open and three with the eyes closed in supported (first six)
and unsupported (last six) standing positions (study 1-3).
The muscle activation onsets and offsets were determined visually
from the rectified EMG. The determination was made without reference
points in order to exclude observer bias (study 1-3).
Dynamic function
Subjects performed sagittal trunk flexion and extension in the standing
position with knees extended and feet 10 cm apart while raw surface
EMG was recorded bilaterally over the paraspinal muscles from the
abovementioned placements. The subjects were instructed to flex their
body to the limit of full flexion and to extend back to the upright
position (forward flexion and extension lasting from 5 to 10 seconds,
Leinonen et al., 2000).
The muscle activation amplitudes were calculated from a 1 sec period
during the flexion movement, full flexion and extension movement,
respectively. The full-trunk flexion and extension activation were
related with flexion activation modified from the previously described
method (Ahern et al., 1988;
Watson et al., 1997).
The range of motion was determined by an accelerometer/goniometer
(Mega Electronics Ltd, Kuopio, Finland) placed on the T6 vertebra.
(study 5)
Lumbar endurance
The back muscle endurance was measured by a previously demonstrated
isoinertial back endurance test (Kankaanpää et al., 1997;
1999). Briefly,
subjects were seated in a back extensor training unit (DBC International,
Helsinki, Finland), which the fixation mechanism (lower limbs and
hips were mechanically bound) focused the dynamic movement to the
upper back. The subjects performed repetitive upper trunk extensions
with a continuous isoinertial load (30 repetitions per min controlled
by a metronome) and the subjects were instructed to hold the tension
at both ends of the movement range. The hyperextension of the spine
was prevented to avoid the provocation of claudication symptoms. The
test was performed until exhaustion or pain, and the endurance time
was measured.
Spectral mean power frequency (MPF) slopes of the first 90 seconds
of the endurance time were calculated by performing a fast Fourier
transformation to assess the muscle fatigue (percentage change of
MPF per minute). The average MPF was calculated from the first 5 seconds
of the performance (initial MPF). The LSS patients (pooled MPF change
and initial MPF at L5-S1 level) were compared with the subjects of
previous studies by t-test [10 healthy controls (Kankaanpää et al.,
1997) and also
with 59 chronic LBP patients who did not suffer any symptoms of LSS
(Kankaanpää et al., 1999)].
(study 5)
Muscle denervation
Electromyography (EMG) of the paraspinal muscles was performed at
the L3 to S1 levels bilaterally with Viking IV EMG equipment (Dantec,
USA) using a concentric needle electrode (Neuroline, 50 x 0.45 mm).
Amplification was set at 50 µV/div and the high and low-pass filters
at 10 kHz and 20 Hz, respectively.
At least 20 insertions were analysed from each multifidus muscle and
the aim of the examination was to detect any abnormal spontaneous
activity indicative of a lower motor neuron disorder and thus as a
sign of denervation. Abnormal activities indicative of denervation
were considered to be fibrillation potentials, positive sharp waves
and complex repetitive discharges (Kimura, 2001).
The analysis was performed on-line (study 5).
Lumbar movement perception
Lumbar proprioception was assessed in a previously described trunk
rotation measurement unit (DBC International Ltd, Helsinki, Finland;
Taimela et al., 1999),
which targeted the rotation on the lumbar/thoracic spine. In the test,
the subject was placed in the device in the seated position, ears
and eyes covered, while the seat was rotated with an angular velocity
of 1o·sec-1 and the subject indicated the initiation
of the movement by releasing a finger switch. The magnitude of the
lumbar rotation was recorded. In addition, the subject was asked to
indicate the direction of movement. The results of five consecutive
trials were pooled. (study 3, 4).
Postural stability
Postural control was measured with a vertical force platform (Smart
Balance Master, NeuroComR, Clackamas, OR, USA), which is
based on the principle introduced by Terekhov (1974).
The load cell signals were recorded by a personal computer with a
sampling frequency of 50 Hz and were low-pass filtered. The postural
control was evaluated during eight 20 sec trials (one two-footed trial
with eyes open and one with eyes closed; six one-footed trials, of
which three were performed while standing with the right foot and
three while standing with the left foot. The center point of force
velocity (CPFV, cm·sec-1) was calculated for each trial
(study 3, 4).
Motor and somatosensory evoked potentials
MEPs were elicited by transcranial and lumbar root stimulation using
a MES-10 magnetic stimulator (Cadwell Laboratories Inc., Kennewick,
WA, USA) with a circular 9-cm-diameter coil producing 2 Tesla. The
motor responses and SEPs were recorded by using standard EMG equipment
(Nicolet Viking I, Dantec, Wisconsin, USA). The responses were high
and low-pass filtered.
MEPs were recorded bilaterally from the anterior tibial muscles using
bipolar Ag-AgCl cup electrodes. The latencies of the MEP after cortical
stimulation (cortical latency, CL) and after lumbar root stimulation
(peripheral latency, PL) were measured from the response with the
shortest latency.
During the SEP procedure, the tibial nerves were stimulated at the
ankle behind the medial malleolus with a surface electrode. The cortical
P40-N50 responses were recorded 2 cm posterior from the vertex referenced
to the forehead with platinum needle electrodes. Peripheral responses
were recorded from the popliteal space with Ag/AgCl cup electrodes.
P40 latencies and P40-N50 amplitudes were measured.
The MEP and SEP latencies were compared with the laboratory reference
values. The P-value was used as a measure of normality and p<0.05
was classified as abnormal. SEP amplitudes <1µV were also considered
as abnormal. (study 4).
Questionnaires and clinical signs
Low back and lower extremity pain intensities were determined by Visual
Analogue Scale (VAS, Scott and Huskinsson, 1976)
and functional disability by Oswestry disability index (ODI, Fairbanks,
1980). The depressive
symptoms were evaluated by Rimon's brief depression scale questionnaire
(RBDS, Keltikangas-Järvinen and Rimon, 1987).
Clinically observed sensory deficit, motor weakness and limited range
of movement (fingertips over the level of the knee during forward
bending) were recorded. (study 2-5).
Statistical analysis
A repeated measures analysis of variance with three (four) within
factors [control of position, level and expectance (and operation)]
was used to analyze the effects of supported position, measurement
level (ES, T12-L1 and MF, L5-S1), anticipation (and operation) for
the short latency response of paraspinal muscles (study 1-3). The
degree of lumbar rotation and CPFV were compared in the patients and
controls by independent samples t-test (study 3), and the effect of
surgery was assessed by paired t-test (study 3). Correlation coefficients
were calculated to assess the associations between questionnaire data,
degree of lumbar rotation, CPFV and paraspinal reflex latencies in
the sciatica patients (study 3) and SEP and MEP (study 4) and muscle
endurance and flexion-extension activation (study 5) in the LSS patients.
Discriminant or multiple linear regression analysis was performed
(study 4, 5). The statistical analyses were performed with SPSS 10.0
software (SPSS, Chicago, IL) and statistical significance was set
as p < 0.05.
|
| RESULTS |
Reliability
of the measurements
Twenty healthy volunteers (study 1) were tested twice within a 1-week
interval to assess the reliability of the postural control, lumbar
movement perception and paraspinal muscle reaction measurements. In
at least 18 (usually 19) of the 20 patients, the two tests yielded
measurements that were within 95% of the deviation for all parameters.
Thus, the reliability of these measurements was acceptable according
to the Bland and Altman method (Bland and Altman, 1986).
Paraspinal muscle responses
Expectation
A short latency response of ~50 ms was observed in paraspinal muscles.
On average the latency was ~3 ms shorter (p = 0.017) during expected
trials and the latency shortened during the first three expected trials
(p = 0.02). Visual expectation decreased also the magnitude of the
response (p < 0.05). Trunk movement was initiated ~35 ms and ~50
ms after the impact of the load at T6 and T12 levels, respectively.
(study 1)
LBP with sciatica
The short latency response for the unexpected upper limb loading was
similar in the patients and controls. The response latency was shortened
by expectation both in healthy controls and sciatica patients in the
controlled standing position, i.e., while the pelvis was supported.
However, expectation did not decrease the response latency of patients
in the uncontrolled position (p=0.014, Figure
3). In other words, there was a significant difference between
the groups with respect to the effect of control of the position on
the effect of expectance of the load. (study 2)
Surgery in sciatica
After discectomy, the reflex latency was shortened
to a greater extent by anticipation more than had been the case prior
to the operation (p = 0.043, Figure
3). Patients with clinically observed motor weakness had less
shortened latencies by expectation at baseline but responded similarly
to the others after surgery but had longer latencies during the unexpected
trials (p = 0.005). (study 3).
Impaired
lumbar movement perception
LBP with sciatica
The threshold to detect a change in the position during lumbar rotation
was ~2.5 and ~1.0 degrees (p = 0.012, Figure
4) in the patients and controls, respectively, and it tended to
decrease in the patients after surgery (p = 0.054, Figure
4). The degree of lumbar rotation and self-reported disability
correlated moderately (r = 0.455, p < 0.05). The improved self-reported
disability correlated with improved movement perception (r = 0.589,
p < 0.05). (study 3).
LSS
The majority of LSS patients (76.9%) reported the movement direction
incorrectly (p = 0.006). Furthermore, these patients consistently
localised the movement sensation elsewhere in their body than in lumbar
region, usually in the shoulder region. The mean SD lumbar rotations
ranged from 7.2° 8.8 to 3.9° 5.9 from the first to the fifth consecutive
trials, respectively (Figure 4).
The LSS patients had a significantly poorer ability to sense a change
in their lumbar position than previously evaluated healthy controls
(p < 0.0002) and LBP patients (p = 0.0005) not suffering from symptoms
of LSS. (study 4).
Postural stability
LBP with sciatica
Patients exhibited larger CPFV values than controls in the two-footed
stance with eyes open (p = 0.022) and with eyes closed (p = 0.001)
and in the one-footed balance test (p = 0.004, Figure
5). Motor weakness (R2=0.355; p = 0.006) was related
with larger two-footed CPFV with eyes open and limited range of movement
(R2 = 0.465; p = 0.001) was related with larger two-footed
CPFV with eyes closed at baseline. Patients with motor weakness (p
= 0.078) and a limited range of movement (p = 0.051) tended to have
larger one-footed CPFV, but there was no difference in the CPFV values
between healthy side and leg with sciatica. The postural control remained
unchanged in the patients after (Figure
5) the microdiscectomy but the improved self-reported disability
correlated (r = 0.524, p < 0.05) with improvements in two-footed
CPFV with eyes open. (study 3).
LSS
The mean ±SD CPFV values were 4.1±1.5 and 3.6±1.3 cm·sec-1
on right and left leg, respectively (Figure
5). Abnormal cortical MEPs were observed in 11 patients (44%)
and abnormal SEPs in 16 patients (61.5%). The association between
motor and sensory conduction and postural stability was inconsistent.
A significant association was found between increased CPFV and prolonged
cortical MEP latencies on the left side but no association was detected
on the right side. No significant associations were found between
abnormal SEPs and CPFV. The associations between impaired lumbar sensation
and increased CPFV as well as lumbar sensation and cortical left side
MEP were of borderline significance. (study 4).
Paraspinal muscle function and endurance
Parapinal muscle denervation
Abnormal findings in the needle EMG of the paraspinal muscles indicative
of denervation, were observed in 18 out of the 22 (81.8%) LSS patients.
Increasing age and lower extremity pain were related with the probability
of abnormal findings in paraspinal needle EMG. Stepwise discriminant
analysis entered the subject's age, lower extremity pain and relative
extension activation at T12-L1 level as independent variables. Wilks'
lambda was 0.184 (p < 0.001) and these variables explained 81.6%
of the variability between the subgroups of the patients with and
without denervation. (study 5).
Flexion-extension function
The average paraspinal muscle activations during full flexion were
~60 and ~65 percent of flexion activation at T12-L1 and L5-S1 levels,
respectively. The full flexion-relaxation was achieved by none of
the patients despite the fact that all but one patient had a range
of flexion movement covering at least 85 degrees. The full-flexion
muscle activation associated poorly with denervation, muscle endurance,
pain and disability, however, the associations between extension activation
and pain and disability were more obvious. The relative extension
activation at T12-L1 level was smaller in the patients with paraspinal
denervation than in those without denervation. Stepwise linear regression
analysis entered lower extremity numbness and paraspinal muscle denervation
as independent variables accounting for ~46% of the variability of
the relative extension activation (R2 = 0.462, p = 0.003).
(study 5).
Lumbar endurance
Paraspinal muscle fatigue (pooled mean SD MPF change at L5-S1 level)
was significantly smaller in LSS patients (-6.8±7.1 %·min-1)
than that observed in previously evaluated healthy subjects (-20.4±11.3
%·min-1) and LBP patients (-21.1±8.7 %·min-1,
p < 0.001) not suffering from the symptoms of LSS. The mean endurance
time (~172 sec) was similar to that of healthy controls. Paraspinal
muscle endurance (MPF change) was not associated with the denervation
of the muscles but the endurance time tended to be shorter (p = 0.059)
in those patients who had denervation. Endurance time, but not the
MPF change, was related with disability and low back and lower extremity
pain. Stepwise linear regression analysis entered lower extremity
pain as an independent variable which explained ~62% of the variability
of the endurance time (R2 = 0.624, p < 0.001). The initial
MPF values of LSS patients (84.4±20.9 Hz) were similar as in the previously
evaluated healthy subjects (79.1 ±14.9 Hz, p = 0.488) and higher than
in LBP patients (71.1±13.2 Hz, p = 0.0009). (study 5).
|
| DISCUSSION |
Main novel findings
This study was aimed at detecting the sensory and motor function of
the lumbar spine and postural control in healthy subjects and two
specific lumbar disorders. The lumbar disc herniation and spinal stenosis
were selected to represent two different stages of the LBP syndrome.
Disc herniation represents an early and more acute stage whereas spinal
stenosis encompasses the late and perhaps the final stage.
Impaired postural control, lumbar movement perception and defective
effect of visual expectation on lumbar muscle control were seen in
patients with sciatica. Movement perception and the defect in anticipatory
processing seem to recover in short term after successful surgery,
but postural control did not recover.
Nearly total loss of lumbar movement perception was observed in lumbar
spinal stenosis, indicative of a clear impairment of lumbar proprioception
associated with LSS. The observations of paraspinal muscle denervation
and disturbed muscle function during dynamic movement were expected
but the exceptionally good lumbar endurance was surprising. The associations
between different pathological findings were inconsistent and not
necessarily related with each other.
Methods
The sciatica patients were selected by a specialist neurosurgeon to
meet the criteria for microdiscectomy surgery. They represented a
specific condition of low back syndrome with sciatica due to a radiologically
confirmed disc herniation and the dropouts did not diverge from the
others. LSS patients were selected by a specialist in physical and
rehabilitation medicine representing a specific condition of low back
syndrome with radicular symptoms due to radiologically confirmed LSS.
The patients of both groups were suffering considerable pain and disability.
The repeatability of paraspinal muscle responses for sudden upper
limb loading, lumbar movement perception and postural stability was
assessed in healthy subjects. The measurements were indicated to be
repeatable and the methods were valid for assessing the studied issues.
However, according to current knowledge it would have been an advantage
to perform all of the measurements in both groups of patients and
also on a larger sample of healthy individuals. The sciatica patients
had a healthy control group comparable with respect to age and height
but LSS patients did not and the use of historical and internal control
limits the value of the results.
Motor control
Anticipation in lumbar motor control
A short latency (~50 ms) paraspinal muscle response for sudden upper
limb loading was observed (study 1), the response being shortened
by visual expectation (study 1). The effect of anticipation can be
explained by feed-forward control, where the visual system alerted
the reflex mechanisms about incoming sensory impulses. Thus, in the
anticipated condition, cortical pre-programming could well be involved
in the response modulation. The response latency was comparable with
the earlier findings (Carlson et al., 1981;
Marsden et al., 1981;
Carpenter et al., 1999;
Hodges et al., 2001).
The shortening of the latency during expected upper limb loading (study
1, 2) has been observed also in a later study (Moseley et al., 2003)
confirming the finding.
The visual expectation did not make the reflex faster in sciatica
patients, in contrast to control subjects in the experiments where
the spine and pelvis were not supported (study 2). This indicates
that prolonged sciatic pain can impair the lumbar feed-forward control.
This is in line with the earlier findings of impaired feed-forward
postural responses of the trunk muscles in CLBP (Hodges and Richardson,
1996; 1998;
Hodges, 2001) and
during experimental muscle pain (Hodges et al., 2003).
The unaffected paraspinal muscle latency during unexpected upper limb
loading differs from the earlier findings of delayed trunk muscle
activation during expected and unexpected upper limb and trunk loading
(Wilder et al., 1996;
Magnusson et al., 1996)
and sudden load release (Radebold et al., 2000;
2001) in CLBP
patients. The difference can be explained by dissimilarity of patient
groups and methodological variation. Wilder et al. (1996)
used computer based wavelet analysis in their assessment of muscle
response latencies and therefore the latencies are not comparable.
During direct trunk loading and load releasing, the triggering mechanism
may be different (study 1). The duration of the symptoms in sciatica
patients (study 2, 3) were usually from three to six months and thus
perhaps the variable changes in trunk muscle control were dependent
on the pathophysiology and duration of the LBP syndrome.
Since the latency of the responses for unexpected stimulus was unaffected,
the limited effect of anticipation in CLBP patients may be due to
impairment in the processing of visual feedback information in the
short-term memory. Short-term memory is not a distinct cortical area,
but an abstract concept representing the co-operation and recruitment
of neural networks in several cortical areas involved in information
processing. It is an information storage process regulating movement
output by processing sensory input between short-term sensory store
and long-term memory (Schmidt and Lee, 1999).
According to the recognition schema theory, the initial conditions,
environmental outcomes, and expected sensory consequences serve as
the basis for movement evaluation, i.e. a schema for movement control
in the short-term memory by sensory information enables motor learning
(Schmidt, 1975;
Schmidt and Lee, 1999).
Therefore, the altered short-term memory is probably the explanation
for the diminished effect of anticipation, revealing the impairment
of feed-forward control system in CLBP patients. It has been hypothesised
that CLBP can impair also the higher level information processing
i.e. functioning of short-term memory. This hypothesis was based on
the observation of missing differences between preferred and non-preferred
upper limbs during hand reaction time test in CLBP patients (Luoto
et al., 1999).
The possible explanations for the difference in the effect of anticipation
between controlled and uncontrolled positions may be the initial condition
where the supports in the controlled position function as reference
points (Schmidt and Lee, 1999).
The controlled initial condition, therefore, enhances the affected
feed-forward control. The possible enhancing effect of these reference
points is in line with the observation that in back healthy subjects
there was a small but significant learning effect to reduce the response
latency during three consecutive trials in the controlled position
but not in the uncontrolled position (study 1).
LBP and depression have been shown to interfere with voluntary motor
control. The impaired feed-forward control values did not correlate
significantly with pain, functional disability and depression scores
in the whole patient group (study 2) but the anticipatory trunk muscle
control does seem to be affected by severe LBP. CLBP and pain-related
depression could be related to the motor behaviour at a higher level
involved in anticipatory behaviour. However, the assessment of a causal
relationship between depression, pain and anticipatory control requires
further studies.
Sensory contributions to motor control
The impaired lumbar movement perception was observed in the patients
with sciatica (study 3), however, the impairment was not as obvious
as that seen in the patients with lumbar spinal stenosis (study 4).
The perception of lumbar movement tended to recover after discectomy,
indicating that at least in sciatica patients, the impaired lumbar
proprioception is a reversible phenomenon (study 3). The impaired
lumbar movement perception may be due to feedback error as a result
of sensory loss or a deficit in information processing or a combination
of both mechanisms. CLBP seems to reorganise the somatosensory cortex
(Flor et al., 1997),
being in line with the idea of reversible pain related dysfunction
of central sensory information processing.
The majority of LSS patients failed to sense the direction of lumbar
rotation correctly and, in addition, they localised the movement sensation
elsewhere than in lumbar region. This is evidence of a peripheral
sensory loss in LSS but does not exclude the possibility of altered
central sensory-motor processing (study 4). The proprioceptive deficit
in LSS was more severe in LSS than in previously observed LBP patients
with no LSS (Taimela et al., 1999).
Since ligament mechanoreceptors (e.g. Sjölander et al., 2002)
and muscle receptors (e.g. McCloskey, 1978;
Sjölander et al., 2002)
are important in propioception and denervation with impaired muscle
function (study 5) and ligament calcification is typically associated
with LSS, the impaired movement sensation in LSS patients was to be
expected. The local loss of lumbar proprioception appears to be more
important in spinal function than the changes in long ascending nerve
tracts evaluated by SEPs.
The current results are in line with the earlier observations of a
large repositioning error in trunk movement in idiopathic LBP (Gill
and Callaghan, 1998;
Brumagne et al., 2000;
Newcomer et al., 2000)
and in lumbar segmental instability (O'Sullivan et al., 2003).
Since muscle spindles have a major role in lumbar proprioception (Brumagne
et al., 1999) and the 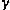 -muscle
spindle system is thought to have a significant function in feed-forward
control (Sjölander et al., 2002),
dysfunction of the muscle spindle system may contribute to both impaired
lumbar proprioception and feed-forward control. -muscle
spindle system is thought to have a significant function in feed-forward
control (Sjölander et al., 2002),
dysfunction of the muscle spindle system may contribute to both impaired
lumbar proprioception and feed-forward control.
Postural control
Impaired postural stability was observed in sciatica (study 3) being
comparable with the previous findings in LBP patients and controls
(Byl and Sinnot, 1991;
Luoto et al., 1998).
In the current study, the two-footed body sway was also increased
in the patients with clinically observed motor weakness and a limited
range of movement. Furthermore, one-footed body sway was not associated
with the side of sciatica in the one-footed test (study 3). The two-footed
postural stability was weaker also with eyes open, but the difference
between patients and controls was more prominent when the eyes were
closed during testing (study 3). This is in agreement with the previous
findings indicating that the impaired sensory input from muscles and
joints is more severely challenged during the absence of visual input
(Byl and Sinnot, 1991;
Mientjes et al., 1999;
Radebold et al., 2001).
The unsuccessful recovery of impaired postural control after surgery
in sciatica is not surprising according to a previous prospective
evaluation in CLBP patients. After active rehabilitation, the impairment
in postural control remained unchanged in the patients with good outcome
but deteriorated further in the patients with poor outcome (Luoto
et al., 1998).
In addition, low performance in a test of standing balance was associated
with future back disorders (Takala and Viikari-Juntura, 2000).
According to the previous studies in conservatively (Balague et al.,
2001; Dubourg et
al., 2002) and
surgically (Dubourg et al., 2002)
treated sciatica patients, the recovery of impaired muscle performance
was not a matter of course even at six months of follow-up. Therefore,
the recovery of postural control may have been still taking place
after three months follow-up. However, many postural reactions are
not triggered by lower leg proprioception, but probably by receptors
in the proximal body segments (Bloem et al., 2000).
Thus, the impaired postural control seems to be related with the information
processing in addition to the lower leg muscle function. A longer
follow-up duration, perhaps with postoperative intervention, would
help in clarifying this issue.
The postural stability usually improves during the repetitive measurements
indicating motor learning. In LSS patients, the postural stability
had a weak negative correlation with lower extremity pain intensity
during standing i.e. those patients with more severe pain seemed to
have better postural stability (study 4). Further analysis showed
that the one-footed postural stability improved during the repetitive
measurements in the patients with less pain, but it seemed to become
even worse in the patients with severe pain. This is further evidence
that pain may impair motor learning.
Postural control is a complex procedure involving integrated sensory
and motor function. An association between poor balance performance
during unstable sitting with eyes closed and delayed trunk muscle
response times during quick force release, reflecting the relationship
between impaired postural control and delayed muscle activation, has
been reported previously in idiopathic CLBP (Radebold et al., 2001).
The current results in sciatica patients are in accordance with those
findings. Additionally, the postural control and muscle response times
correlated with impaired proprioception (study 3). Impaired postural
control was associated with impaired lumbar perception both in sciatica
and LSS patients (study 3, 4). In LSS patients, according to the evoked
potential findings, the one-footed postural control seemed to be more
dependent on motor than sensory conduction between the lower limbs
and the central nervous system (study 4).
Function of lumbar muscles
Impaired lumbar muscle function and denervation were frequently observed
in LSS patients. The lumbar endurance time of LSS patients was associated
with low back and lower extremity pain but not with power frequency
change. The limiting factor of endurance in some LSS patients was
not the paraspinal muscle fatigue but the pain intensity, especially
the intensity of radicular symptoms (study 5). I also attempted to
measure lumbar endurance in sciatica patients, but because half of
the first eight patients were unable to perform the test due to intolerable
pain, further analyses were discontinued.
The value of dynamic lumbar endurance assessments and power frequency
analysis as a measurement of muscle function must be questioned in
patients with muscle denervation and radicular symptoms (study 5).
However, the endurance test could be useful in monitoring the functional
ability and the fatigue resistance of paraspinal muscles in LSS patients
indicate indirect evidence for selective type 2 muscle fiber atrophy
possibly associated with LSS (study 5). These results are in accordance
with an earlier proposal of the low diagnostic value of the isometric
trunk strength test (Rantanen, 2001).
That test was indicated to be a potentially hazardous for high-risk
patients due the cardiovascular stress (Rantanen, 2001),
which could be particularly dangerous in LSS patients who often are
elderly people.
The paraspinal muscle dysfunction during flexion-extension movement
was similar in LSS patients (study 5) as previously observed in CLBP
patients (Ahern et al., 1988;
Sihvonen et al., 1991;
Shirado et al., 1995;
Sihvonen et al., 1997)
and during experimental pain (Zedka et al., 1999).
This was evident as increased paraspinal muscle activity when acting
as an antagonist and decreased activity when acting as an agonist
(study 5). The different recruitment patterns found in CLBP patients
increased spinal stability and therefore may represent a beneficial
compensation mechanism (van Dieen et al., 2003a;
2003b). Decreased
extension activation but not the increased flexion-relaxation activation
was related with the denervation and subjective low back and lower
extremity symptoms and were not correlated with each other (study
5). Thus, these could be independent phenomena reflecting different
changes in motor control properties.
Abnormal paraspinal needle EMG findings indicate that denervation
of paraspinal muscles was frequent in LSS patients (study 5). In previous
studies, a denervation of multifidus muscles was often observed in
sciatica patients (Mattila et al., 1986;
Rantanen et al., 1993).
Although the muscle receptors seem be important in lumbar perception
(Brumagne et al., 1999;
2000, Taimela et
al., 1999), the
impaired lumbar perception did not have any direct relationship with
the muscle denervation findings in LSS. However, the denervation findings
often represent only a limited area of the paraspinal muscles, which
may explain their poor relationship with both lumbar muscle endurance
and proprioception. Since the extensive spinal degeneration and abnormal
paraspinal muscles are common in LSS the dorsal ramus neuropathy (Sihvonen,
1995) may be associated
also with LSS and therefore their evaluation would be beneficial in
the assessment of pathophysiology of LSS.
Clinical significance and hypothesis for further investigation
The chronic pain may modulate neuronal activity at the spinal, intermediate
and cortical levels (Flor et al., 1997;
Watkins and Maier, 2002;
Juottonen et al., 2002;
Farina et al., 2003)
and it may alter both central tactile and motor processing (Juottonen
et al., 2002).
The impaired motor control can be restored by rehabilitation procedures.
In rehabilitation, the aim is to enhance the motor performance. In
painful conditions, the central information processing can be disturbed
by the sensation of pain. This may lead to disuse of muscles by fear-avoidance
behaviour (Vlaeyen et al., 1999;
Vlaeyen and Linton, 2000).
During exercise, the brain receives alternative information in addition
to pain signals, this probably being explained by gate control theory,
decreased sensitivity of receptive structures or CNS remodelling.
Thus, this pain-related CNS remodelling may be reversed by rehabilitation
(Flor, 2003). This
may lead to relief of pain and break the vicious cycle of pain, disuse
and de-conditioning. The apparently partly reversible phenomenon of
impaired feed-forward control in sciatica (study 2, 3) indicate a
possible site of motor control to focus rehabilitation of LBP patients.
The effect of rehabilitation may elicit better neuronal function as
well as improved muscle properties. The cognitive effect may at least
in certain situations be even more important than the effect at the
muscle level. The crucial factor is to enhance muscular function by
improving the muscle control. After successful rehabilitation of CLBP
patients (Mannion et al., 2001b)
no change in histologically analysed structural muscle anatomy was
observed (Kaser et al., 2001)
and the changes in muscle performance were mainly related with psychological
factors (Mannion et al., 2001a;
2001b), however,
the histological changes seem to appear slowly (Rissanen et al., 1995).
The current study supports the idea that co-ordination and muscle
control are perhaps more important in the rehabilitation of LBP patients
than muscle strength.
The functional brain imaging has provided new insight into the anatomical
structures and physiological functions of pain processing (Flor et
al., 1997; Peyron
et al., 2000).
In addition to the imaging of neuronal function, other methods used
in cognitive neuroscience could be advantageous in assessing the chronic
pain. The current results point to impaired sensory function and information
processing being associated with both LSS and sciatica.
In future studies the representative cohorts of LBP patients with
various spinal pathologies should be tested with the full measurement
repertoire in prospective settings. This kind of simultaneous testing
would clarify the associations between the findings. The prospective
setting with an adequate follow-up time would clarify the still remaining
question, which phenomena are the cause and which are the consequence
of the problem. Furthermore, it is important to clarify which phenomena
are reversible and through which kind of intervention.
This work does not alter the current clinical practice but does broaden
our view of the LBP syndrome and in the terms of WHO can provide new
insights into sensory and neuromusculoskeletal functions associated
with lumbar disorders and has provided novel hypotheses which can
be evaluated in further experiments.
| CONCLUSIONS |
|
Impaired
anticipation related lumbar motor control, proprioception
and postural control were observed in LBP patients with sciatica.
Anticipation related lumbar control and proprioception improved
after surgery but postural stability remained unchanged. These
results indicate that, in addition to lumbar nerve root irritation,
there seem to be changes of the central motor control in LBP
with disc herniation and that the recovery of these impairments
is not simply a matter of course.
Extensive loss of lumbar proprioception, lumbar paraspinal
muscle denervation and impaired muscle function were observed
in lumbar spinal stenosis. These findings indicate that there
appears to be not only notable structural pathologies in the
lumbar spine but also clearly abnormal lumbar function in
LSS.
|
| ACKNOWLEDGMENTS |
|
This
work was carried out in the Department of Physical and Rehabilitation
Medicine, Kuopio University Hospital and in the Department
of Physiology, University of Kuopio in collaboration with
the Departments of Neurosurgery and Clinical Neurophysiology,
Kuopio University Hospital during the years 1999-2003.
I express my sincere gratitude to my principal supervisor
Docent Olavi Airaksinen, M.D., Ph.D., Head of the Department
of Physical and Rehabilitation Medicine, Kuopio University
Hospital for enthusiastic support and guidance during this
work. In addition to the never failing encouragement, he provided
the necessary facilities for this study.
I am greatly impressed for the endless ideas of my supervisor,
Professor Osmo Hänninen, M.D., Ph.D., Head of the Department
of Physiology, University of Kuopio. I thank him for introducing
me to the world of science and the encouraging discussions
at any time of the day.
I am deeply grateful to my supervisor Docent Simo Taimela,
M.D., Ph.D., CEO & Medical Director of DBC International
Ltd., for his special expertise and essential help in this
work. He always pointed out the key issues and confirmed the
finalisation of the studies.
I express my warm thanks to Markku Kankaanpää, M.D., Ph.D.,
for introducing me this work and the constructive guidance
especially in the initiation of the study.
I owe my sincere thanks to Docent Arto Herno, M.D., Ph.D.,
for interesting discussions and recruiting and managing the
stenosis patients, Matti Luukkonen, M.D., for recruiting and
managing the disc herniation patients.
I thank my other co-authors Sara Määttä, M.D. and Professor
Juhani Partanen, M.D., Ph.D., Head of the Department of Clinical
Neurophysiology, for interesting discussions and expertise
in the field of neurophysiology and Martti Kansanen M.D.,
Ph.D. from the department of Otorhinolaryngology.
I thank Jari Arokoski, M.D., Ph.D., for interesting discussions
and Sakari Savolainen, M.D., Ph.D., and Veli Turunen, M.D.,
for introducing me the spinal surgery. I owe sincere respect
to Ville Westerlund and Tommi Kääriäinen for fruitful thoughts
and continuing this work.
I wish to express special thanks to the entire staff of Departments
of Physical and Rehabilitation Medicine, Physiology and Neurosurgery.
I thank all persons who participated to this study.
I thank the official referees of this study, Docent Heikki
Hurri M.D., Ph.D., and Docent Uolevi Tolonen, M.D., Ph.D.,
for they thorough review and constructive criticism.
I thank Pirjo Halonen, M.Sc., for expert statistical assistance,
Heikki Aalto, Ph.D., from the Department of Otorhinolaryngology,
Helsinki University Central Hospital for his valuable help
in the body sway analysis, Ewen MacDonald, Ph.D., for correcting
the language of both this summary and the original publications.
I owe a special thank to Mega Electronics Ltd., especially
Juha Kylliäinen, M.Sc., Rainer Mustonen, M.Sc. and Kari Tiihonen,
M.Sc., for their indispensable technical support.
I am grateful to all my co-workers and friends for their support
and encouragement.
I express my deep feelings and gratitude to my parents Marja
and Simo Leinonen and my parents in law Pirjo and Risto Hirvonen
for loving support.
Finally, my deepest and warmest loving thank I give to my
dear wife Leena whose love and support has kept me alive during
these years and to whom I dedicate this thesis.
The
research was financially supported by grants from Kuopio University
Hospital, the Ministry of Education and Academy of Finland
(TULES Graduate School), the Finnish Medical Society Duodecim,
Savo Foundation for Advanced Technology and Finnish Cultural
Foundation.
Kuopio, October, 2003
Ville
Leinonen
|
|
| AUTHOR
BIOGRAPHY |
Ville LEINONEN
Employment: Resident, Department
of Neurosurgery, Kuopio University Hospital, Kuopio, FIN
Degrees: Licentiate of Medicine (MD), 2002, Univ. of
Kuopio, FIN
Doctor of Medical Sciences (DMS), 2003, Univ. of Kuopio, FIN
Research interests: Low back pain
Email: Ville.Leinonen@kuh.fi
|
|
|
|
|