| METHODS
OF BODY COMPOSITION ASSESSMENT |
Underwater weighing
The measurement of body density is often referred to as a golden standard
for body composition assessment; despite it is only a 2-C model. The most
commonly used method for assessing total body density is UWW, which requires
the subject to be submerged in water. Then the residual lung volume is
measured, the most commonly used method is oxygen dilution with a closed-circuit
spirometer system. The volume of water displaced or the weight of the
subject underwater, compared to the subject's normal weight, are used
to calculate the density of the whole body. The result is then corrected
by residual lung volume (Siri, 1956;
Brozek, 1966).
The UWW methods were developed mainly as a means to measure body volume
to assess body fatness (FM and/or F%). Even if the body weight and volume
could be measured without error, there would still be considerable uncertainty
regarding the individual's body fatness estimate due to normal variations
in body hydration, protein, and mineral content. It has been estimated
that the total error for body fatness is ~3-4% of body weight for individuals
(Heymsfield et al., 1989).
Thus, it has been recommended that without correction for variation in
the water and mineral content of FFM, densitometry should not be used
as a reference method for heterogenic population.
Dilution method
Dilution method with labelled water is an accurate method for total body
water (TBW) measurement. It is performed by introducing orally or intravenously
a certain substance (a tracer) like deuterium oxide (D2O),
oxygen-18, or tritium (radioactive; not recommended) into the human body,
and then collecting two body fluid samples (blood, urine, or saliva),one
before introducing the tracer and second after an equilibration time of
~2-4 hours. Then the substance (tracer) is equilibrated with water in
the subject's body, and the density of the substance is analyzed. Dilution
takes many hours of measurement and cannot be measured repeatedly. However,
because it is rather accurate, it is used to measure body water in research
purposes (Ellis, 2000).
Dual energy x-ray absorptiometry
Dual energy X-ray absorptiometry (DXA) is the most used imaging method
of body composition assessment. It is mostly used for bone density assessment
for osteoporosis diagnostic purposes and clinical bone research, but also
whole body composition assessment by DXA has become a popular method.
Hence, DXA is available in most of the bigger hospitals and research centers.
When an X-ray or photon source is placed on one side of an object, the
intensity of the beam on the opposite side of the object is related to
its thickness, density, and chemical composition. This attenuation phenomenon
is also dependent on the energy of the incident photons and is dominated
by two principles at low energies: the photoelectric effect and Compton
scattering. The attenuation response is nonlinear, such that for a homogeneous
material, it can be described by the exponential equation. If the absorber
is composed of two or more materials, then the composite is the weight
sum of the individual mass attenuation coefficients, each weighted for
its fractional contribution to the total mass (Heymsfield et al., 1997).
The attenuation through bone, lean tissue, and fat is different, reflecting
their differences in densities and chemical composition. With increasing
photon energy, the differences in the attenuation properties for these
tissues decrease. Thus, if the relative intensity of the transmitted beam
can be measured, and the mass attenuation coefficients are accurately
known, estimates of the bone mass and overlaying soft tissue mass can
be calculated. This 2-C model is also used when the beam passes through
body regions without bone. In this case, the appropriate attenuation coefficients
are those for fat and lean tissues, respectively (Heymsfield et al., 1997).
It should be evident that DXA is composed of two separate sets of equations,
each used to describe a 2-C model. Dual-energy X-ray absorptiometry does
not provide three independent measurements, even though three body composition
values [bone mineral content (BMC), lean tissue mass (LTM), and fat mass
(FM)] are reported (Figure 4.). To
accomplish this, the manufacturers must assume that the composition of
the soft tissue layer overlaying bone has the same fat-to-lean ratio as
that for non-bone pixels in the same scan region. In the case of the whole
body scan, ~40-45% of the pixels are classified as containing bone. The
remaining pixels are used to estimate the body's fat-to-lean ratio; this
value is applied to the soft tissue component in the adjacent bone pixels.
Thus, the relative lean-to-fat composition of the total soft tissue mass
is based on sampling only one-half of the whole body (Mazess et al., 1990).
Some investigators have expressed their concern about measurement bias
related to the impact of a significant change in the hydration of the
lean tissues. The latter concern, however, has recently been theoretically
shown not to significantly alter the estimates for the bone, lean, or
fat mass (Pietrobelli et al., 1998b).
Computed tomography
Computed tomography (CT) technique uses X-rays that are collimated to
provide a fan-shaped beam that is passed through the body, while an array
of detectors is on the opposite side of the subject to detect the transmitted
radiation. The X-ray source and detector assembly are rotated as a single
unit around the subject covering a full 360°. Alternatively, some devices
have a full circle of detectors, and only the X-ray source is rotated.
At each degree of rotation, the transmitted intensity is recorded for
each detector, providing information about the internal structures along
that beam circle (Baumgartner et al., 1988).
Many reconstruction algorithms have been demonstrated to produce a cross-sectional
image of the body region. This basic anatomical image contains information
of the tissue density at each pixel. This information together with the
anatomical location of the pixel in the image can be used to identify
it as adipose, muscle, skin, visceral, or bone tissue. Reconstruction
of total body mass and separate organ masses based on scans along the
length of the body at 10-cm intervals has been shown to have excellent
accuracy (<1% error) and precision (<1%). These reconstructed CT images
can be assigned to level 4 or tissue systems level of the multi-component
model. The CT images can also be used to separate the total adipose tissue
mass into its subcutaneous and visceral components, or the lean tissues
into skeletal muscle and visceral or organ mass. Likewise, bone can be
identified as cortical or trabecular in nature on the basis of density
(Baumgartner et al., 1988;
Rossner et al., 1990;
Ross et al., 1991).
The major disadvantage with CT is the radiation dose required per slice
for scanning. However, if the pixel resolution required for routine CT
scans can be reduced, then the dose can be significantly reduced. Recent
studies have shown that a dose can be reduced to 1/25 that of a routine
clinical CT scan dose (Baumgartner et al., 1988;
Rossner et al., 1990;
Ross et al., 1991; Goodpaster
et al., 2000).
Magnetic resonance imaging
The strength of the earth's magnetic fields is very weak, despite that,
atoms and molecules in the body are in random orientations. However, when
the body is placed in a strong magnetic field, some nuclei tend to align
with or against the magnetic field. Hydrogen protons (1H),
in particular, have a high affinity for alignment with the magnetic field.
Some other atoms (13C, 19F,
23Na, 31P, and 39K)
also show similar properties, but at a lower response than for hydrogen
atoms. Although only a small percentage of nuclei will be aligned, the
number is sufficient to detect a change in their orientation when the
magnetic field is removed or altered.
The frequency at which nuclei for each element will flip is called the
Larmor frequency. When radiofrequency (RF) energy, at the Larmor frequency,
is applied perpendicular to the direction of the magnetic field, the nuclei
will absorb this energy and change its alignment. When the RF field is
turned off, the nuclei will lose their alignment and release the stored
energy. The intensity of this signal can be used to measure the number
of hydrogen nuclei of the tissue. This process can be repeated at each
position along the length of body until the whole body is mapped and cross-sectional
images at each slice can be generated. Magnetic resonance imaging (MRI)
is successful because hydrogen, found mainly in water, is one of the most
abundant non-bound elements in the body. For other elements, their concentrations
in the body are lower and the Larmor frequency changes, thus requiring
an increased magnetic field strength if imaging is to be considered (Abate
et al., 1994; Mitsiopoulos
et al., 1998).
If the hydrogen densities of adipose and lean tissues were markedly different,
then it would be possible to develop images based solely on their number
of nuclei. To see the contrast between lean and fat tissues, a second
feature of the nuclei, called relaxation time (T1), is used. This is the
time it takes for the nuclei to release the RF-induced energy and return
to a random configuration. The T1 for protons in fat is much shorter than
that for protons in water. This contrast can be maximized by adjusting
the time interval of the RF pulse and the time to detect the induced signal.
The total process is often referred to as a pulse sequence or spin-echo
sequence (Ross et al., 1991;
Baumgartner et al., 1992;
Ross et al., 1992; Abate
et al., 1994; Mitsiopoulos
et al., 1998; Ross et
al., 2000).
Neutron activation analysis
A substantial paradigm change in the study of body composition occurred
with the development of in vivo neutron activation analysis (NAA) technique.
These procedures allowed for the direct elemental analysis of the living
human body. Alternate body composition techniques such as CT, MRI, DXA,
BIA, and dilution method provide estimation related to tissue density
or volume, but not chemical content. For this reason, the multi-component
models based on NAA have become the most preferred reference for evaluation
and/or calibration of the alternate methods. Virtually all the major elements
of the body can be assayed in vivo: hydrogen, oxygen, carbon, nitrogen,
calcium, phosphorus, sodium, chlorine, and potassium (via 40K
counting). Accuracy of NAA is at least as good as that obtained using
classical "wet chemistry" techniques. Although NAA facilities have been
in use for more than 30 years, a direct comparison of results between
two systems has been reported only recently. A major disadvantage of the
NAA technique is that most of the dose is delivered to the body without
the production of a useful signal. Probably one of the major concerns
restraining a more general use of NAA technique is the association to
radiation exposure. (Beddoe and Hill, 1985;
Pierson et al., 1990;
Wang et al., 1993; Morgan,
2000; Wang et al., 2002).
Bioelectric impedance analysis
The property of tissues, and the whole body, to conduct an electric current
has been known for more than a hundred years. Electricity flows through
tissues that contain water in the human body. When electric signals are
sent to our body, electric currents flows through the most conductive
tissue. Depending on the amount of body water, the width of the passage
of electricity flows is determined. The aqueous tissues of the body, due
to their dissolved electrolytes, are the major pathways of an electrical
current, whereas body fat and bone have relatively poor conductive properties.
Bioelectric impedance analysis (BIA) measures body water based on this
principle.
For the BIA measurement, a low alternating current is conducted through
the outer pair of electrodes, while the inner pair of electrodes from
which the impedance is measured measure the drop of voltage. To convert
this information to a volume estimate, two basic assumptions are used.
Firstly, the body can be modelled as a cylindrical conductor with its
length proportional to the subject's height (Ht). Secondly, the reactance
component (X) contributing to the body's impedance (Z) is small, such
that the resistance component (R) can be considered equivalent to body
impedance. When these two assumptions are combined, it can be shown that
the conducting volume is proportional to the term Ht2/R,
called the impedance index. In the classical BIA method, it was hypothesized
that a human body is a cylinder like the figure below (Figure
5), and BIA measures total body water (TBW). The amount of water is
equal to the volume of the cylinder. With BIA, the cell membrane and fat
tissue show high impedance with electric current. Electrolytes dissolved
in the water of lean body tissue provide good conductivity.
Since BIA is estimating the water content of the body compartments, the
stable state of tissue hydration is crucial for the reliable BIA measurement.
Furthermore, BIA principle implies that the meal and the weight of food
in intestines (Fogelholm et al., 1993;
Slinde and Rossander-Hulthen, 2001),
clothing, skin temperature, and blood flow (Caton et al., 1988;
Liang and Norris, 1993;
Gudivaka et al., 1996;
Liang et al., 2000),
and so forth affects the accuracy of measurement.
From total body water, body fat mass can be estimated. After age, height
and weight are taken into account the body water is estimated. Lean body
mass is in proportion to total body water. The estimation is based on
a constant relationship of 72.3% water in LBM (Wang et al., 1999).
When LBM is subtracted from weight, body fat mass is calculated, 2-C model.
Body fat mass is typically calculated with a regression analysis equation
directly from the impedance index.
The bioelectrical impedance method is easy to use and can be readily
repeated. At present, BIA is probably the most frequently used method,
due mainly to the relatively inexpensive cost of the basic instrumentation,
its ease of operation, and its portability.
Single-frequency bioimpedance analysis
Traditionally the BIA measurements are performed using four electrodes:
usually two are attached at the wrist and two at the ankle and measurement
is performed in supine position. Typically a single frequency current
of 50 kHz is used. At 50 kHz the body's impedance has both resistive and
reactive components. The reactive component is assumed to be related to
the portion of the current that passes through cells, which act like capacitors
that shift the voltage, and current out of phase.
Multi-frequency bioimpedance analysis
Multi-frequency bioelectrical impedance analysis (MFBIA) or sometimes
bioelectrical impedance spectroscopy (BIS) has been mainly developed for
improving the accuracy of BIA and also for assessing the ratio of intracellular
water (ICW) and extracellular water (ECW). The technique is based on the
hypothesis that lower frequencies (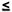 50
kHz) flows within extracellular compartment, whereas higher frequencies
(>200 kHz) could pass trough cell membrane and hence measure the intracellular
space (Figure 6). Moreover, when
the frequency of the electrical signal is changed, the direction of the
electricity can be changed. Using this principle, intracellular fluid
and extracellular fluid can be distinguished and measured separately.
Normally, MFBIA is used at frequencies from 1 kHz ~ 1 MHz (Cha et al.,
1995; Ellis et al.,
1999b; Gudivaka et al.,
1999; Tagliabue et al.,
2001). 50
kHz) flows within extracellular compartment, whereas higher frequencies
(>200 kHz) could pass trough cell membrane and hence measure the intracellular
space (Figure 6). Moreover, when
the frequency of the electrical signal is changed, the direction of the
electricity can be changed. Using this principle, intracellular fluid
and extracellular fluid can be distinguished and measured separately.
Normally, MFBIA is used at frequencies from 1 kHz ~ 1 MHz (Cha et al.,
1995; Ellis et al.,
1999b; Gudivaka et al.,
1999; Tagliabue et al.,
2001).
Segmental bioimpedance analysis
Segmental analysis of body composition can be understood in two different
bases. Firstly, by measuring a certain body segment and thereafter from
the result assessing the total body composition. Secondly, by measuring
all different body segments separately one could improve the accuracy
of total body composition assessment. (Figure
7) Typical model for segmental assessment by BIA, also known from
DXA models, is to divide body as upper and lower extremities and trunk.
This is the segmental model taken in consideration hereafter in this thesis
(Chumlea et al., 1988;
Chumlea et al., 1990;
Organ et al., 1994).
Segmental multi-frequency bioimpedance analysis
By combining segmental and multi-frequency bioimpedance methods there
could be found some benefits in accuracy and possibility of determining
body shape variation in individuals. Moreover, the need for some background
information such as age or gender for the equation used for BIA, body
composition assessment may become less dominant in multiple regression
analysis. Pietrobelli and Tagliabue with groups have reported advances
in appendicular LBM assessment by SMFBIA (Pietrobelli et al., 1998a;
Tagliabue et al., 2001).
While Bedogni and Cha have described TBW assessment advances by SMFBIA
(Cha et al., 1995; Bedogni
et al., 2002). This method
is further studied in this thesis.
Air displacement plethysmography
In recent years, the UWW technique has begun to be replaced by air-displacement
plethysmography, where the subject is immersed not in water but in a closed
air-filled chamber.
The system consists of two chambers: one for the subject and the other
serving as a reference volume (Figure
8). With the subject in one chamber, the door is closed and sealed,
the pressure increased slightly, and a diaphragm, separating the two chambers,
is oscillated to slightly alter the volumes. The classic relationship
of pressure versus volume, at a fixed temperature, is used to solve for
the volume of the subject chamber.
An advantage of this technique compared with the UWW measurement is that
the subject does not have to be submerged in water; however, all of the
technical limitations related to the true volume that were noted for the
UWW method remain. These instruments presently are designed for adults
and will require significant modifications and improvements if the technique
is to become useful for monitoring smaller subjects, including infants
(Dempster and Aitkens, 1995;
McCrory et al., 1995;
McCrory et al., 1998;
Levenhagen et al., 1999).
Skinfold thickness
When squeezing the triceps of an obese person, we can perceive that it
is thicker than that of a muscular person. This is because the subcutaneous
fat is thicker. Considering the fact that over 50% of body fat is under
the skin, thickness of subcutaneous fat in the triceps, thighs and abdomen
can be measured with a round shaped ruler. The skinfold measurement (subcutaneous
fat thickness measuring method) is used for calculating total body fat,
by measuring thickness of subcutaneous fat. Skinfold measurement is done
under the assumption that 50% of subcutaneous fat is body fat. If the
body fat distribution varies within individuals, it affects the accuracy
and the measurement results. Therefore, reliability of this method is
low (Wagner and Heyward, 1999).
Near-infrared measurement When an object is exposed to infrared light,
the object absorbs or reflects the light according to its chemical structural
properties. All organic materials (i.e. fat or protein) absorb light in
unique portions of the spectrum. Futrex, Inc., MD, USA has developed a
device to analyze human body composition by this technique. It estimates
body composition by analyzing the spectrum of near infrared light reflected
from the skin and underlying tissue (Conway et al., 1984;
Elia et al., 1990; McLean
and Skinner, 1992).
The Futrex device emits near infrared light at very precise frequencies
(938 nm and 948 nm) into body-frequencies at which a body fat absorbs
the light and the lean body mass reflect the light. In essence, it is
measuring how much light is emitted from the light wand and how much light
is reflected back into the light wand. This measurement provides an estimation
of the distribution between the body fat and lean body mass (Conway et
al., 1984; Elia et al.,
1990; McLean and Skinner,
1992).
Infrared measurements from the biceps have been the primary site shown
to correlate best with a criterion method However, it overestimates the
fat of a thin person and underestimates the fat in an obese person compared
to underwater weighing, skinfold measurement or BIA (Conway et al., 1984;
Elia et al., 1990; McLean
and Skinner, 1992).
Circumferences and other measurement methods
It is generally accepted that not only excess weight, but also the distribution
of body fat, is associated with several diseases. In particular, abdominal
obesity, indicated by a high waist circumference, waist-to-hip ratio (WHR),
and abdominal sagittal measurement have been shown to predict diseases
like hypertension, coronary heart disease (CHD), non-insulin dependent
diabetes mellitus (NIDDM) and stroke, and these measurements have been
shown to correlate with other cardiovascular risk factors and increase
mortality independent of body mass. It has been suggested that this excess
risk with central obesity is primarily due to metabolic alterations caused
by intra-abdominal fat deposits (Molarius and Seidell, 1998).
Body mass index (BMI) is also an independent marker of body composition
and therefore available method for assessing obesity and overweight related
diseases (Lahti-Koski, 2001).
Waist circumference
Waist circumference (WC) is a very strong predictor for abdominal obesity
related diseases. The recommended procedure for the measurement of the
WC is following: Subject standing with light garments and waist is measured
at the level midway between the lower rib margin and the iliac crest,
with the participant breathing out gently. The measurement is usually
rounded to the nearest 0.5 cm. The cut-off points of WC are classified
and recommended by WHO (WHO, 2000;
Janssen et al., 2002).
Recent evidence suggests that WC alone may be a better indicator of abdominal
visceral fat (Rankinen et al., 1999)
and a predictor of abdominal obesity related diseases (Molarius and Seidell,
1998; Molarius et al.,
1999a; Molarius et al.,
1999b; Nordhamn et al.,
2000) and mortality
(Visscher et al., 2001)
than WHR or BMI.
Waist-hip ratio
Waist-hip ratio (WHR) is probably the most used method for assessing abdominal
fat distribution so far. The recommended procedure for the measurement
of the WHR is following: Subject standing with light garments waist is
measured at the level midway between the lower rib margin and the iliac
crest, with the participant breathing out gently. Hip is measured as the
maximum circumference over the buttocks. Both measurements are rounded
to the nearest 0.5 cm. The WHR is calculated by dividing waist circumference
with hip circumference. The suggested cut-off points of WHR are varying
between 0.80 and 0.90 for female and 0.94 and 1.00 for male, respectively
(Molarius and Seidell, 1998;
Lahti-Koski, 2001).
Sagittal abdominal diameter
Sagittal abdominal diameter (SAD) is a less used assessment method among
anthropometrical methods. Despite, it has been studied to be one of the
strongest predictors of overweight related diseases. Besides, SAD has
one of the highest reliability among similar measurements and it is rather
easy to perform with inexpensive ruler. SAD is measured with the subject
in a supine position. It can be done both with the legs stretched and
bent but legs bent have showed some better confidence to predictability.
The perpendicular distance between the plane of support and the highest
point of the abdomen is measured and read to the nearest 1 mm. (Nordhamn
et al., 2000; Ohrvall
et al., 2000) The major
problem with SAD is that there is a lack of cut-off values. For these
reasons, further research about SAD should be conducted very soon.
Body mass index
Body mass index (BMI) is probably the most used assessment index of obesity.
BMI is calculated by dividing weight (kg) with squared height (m). It
is important to understand that BMI is not a measure (kg·m-2)
it is just an index. The biggest source of error comes from subjects with
high muscle mass in other words BMI fails to distinguish between lean
body mass and fat (Lahti-Koski, 2001).
The classification of body composition by BMI, recommended by WHO, is
presented in Table 4.
The objective of this study was to evaluate segmental multi-frequency
bioimpedance method (SMFBIA) in body composition assessment. The aim was
to cross-validate SMFBIA against under water weighing (UWW) and whole
body dual energy x-ray absorptiometry (DXA).
1. The primary outcome was to compare fat mass (FM), fat free mass (FFM)
and fat percentage (F%) from all three methods.
2. The secondary objective was to compare segmental distribution of FFM
by SMFBIA and whole body DXA.
|
|